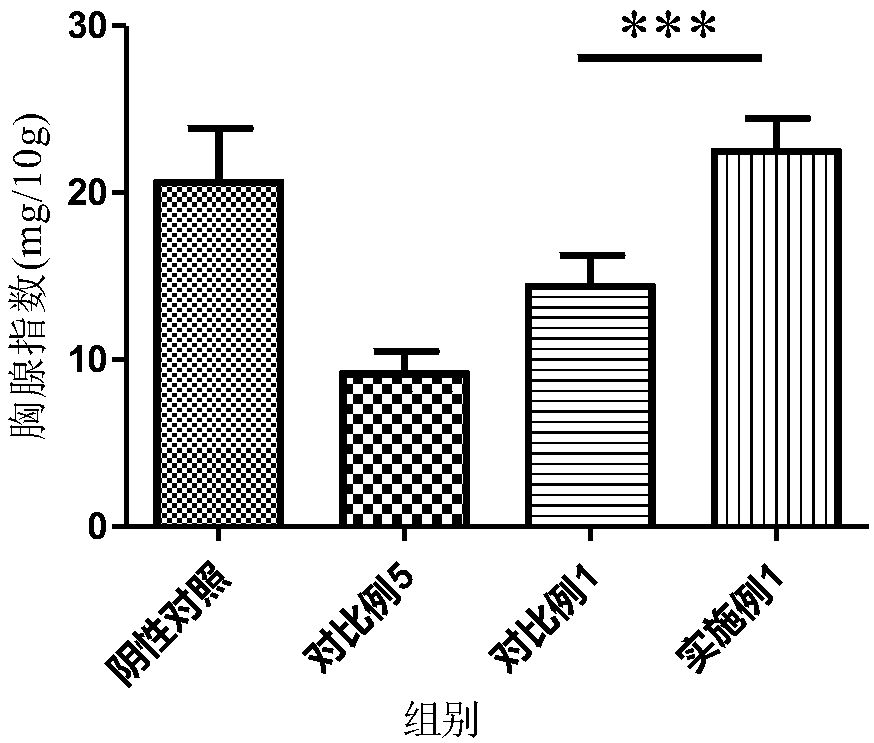
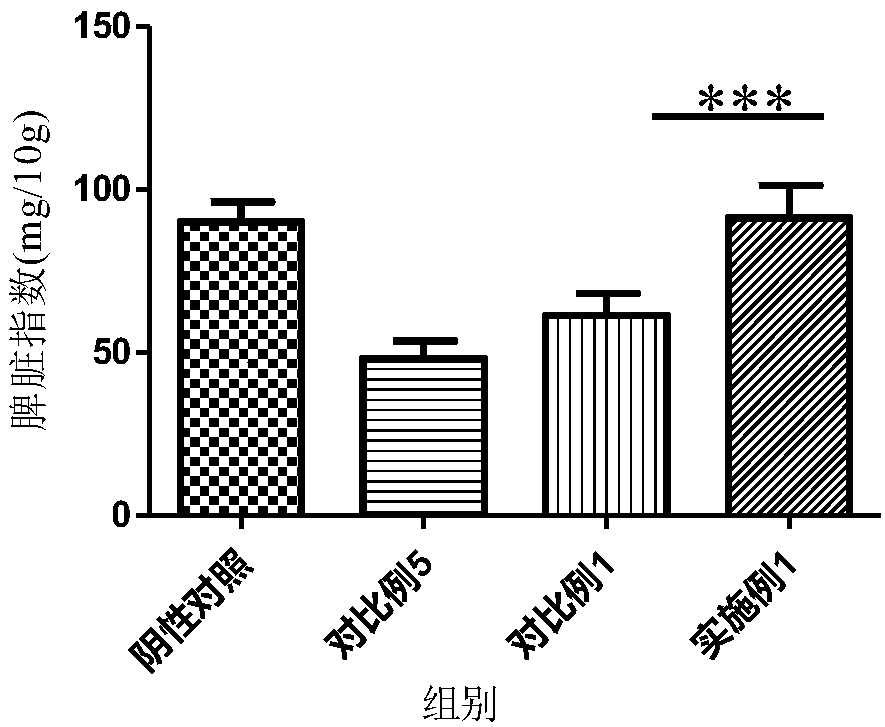
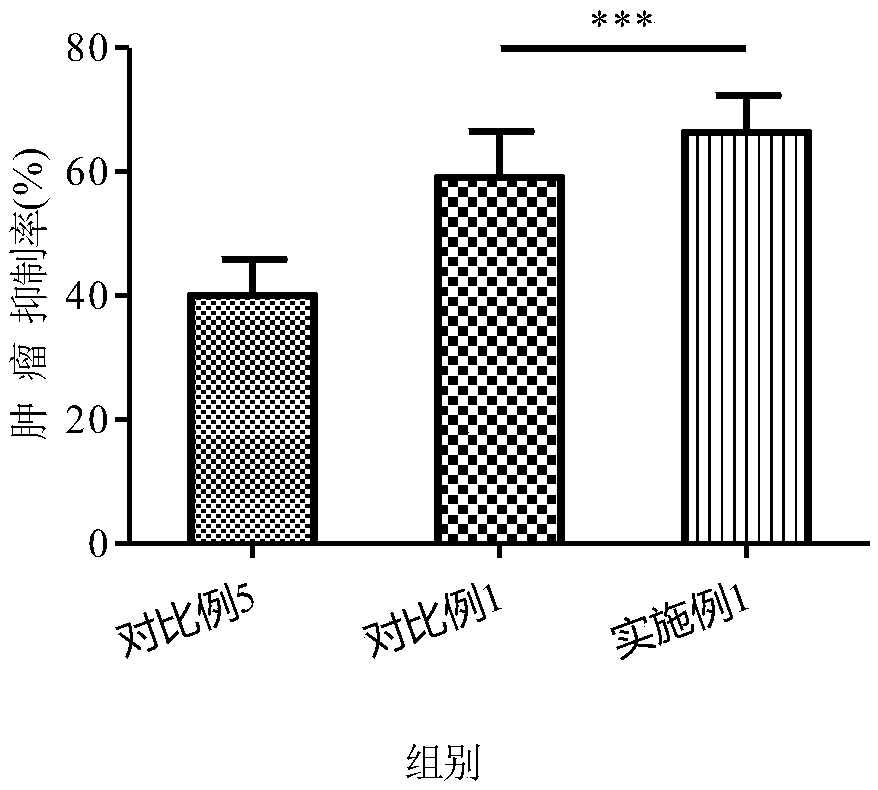
Tamoxifen citrate liposome and preparation method thereof
A technology of tamoxifen and citric acid, which is applied in the field of medicine, can solve the problems of failing to reduce toxic and side effects, achieve the effects of improving solubility and bioavailability, reducing drug toxicity, and prolonging drug action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The invention provides a kind of preparation method of tamoxifen citrate liposome, comprising:
[0026] Tamoxifen citrate, phospholipids and cholesterol are dissolved in a solvent, and a liposome film is prepared by gradient vacuum evaporation; the gradient vacuum evaporation is specifically:
[0027] -0.01~-0.02Mpa rotary evaporation for 20~40min; -0.04~-0.05Mpa rotary evaporation for 20~30min; -0.06~-0.07Mpa rotary evaporation for 10~20min; -0.08~-0.1Mpa rotary evaporation for 3~5min;
[0028] The liposome film is hydrated and filtered to obtain tamoxifen citrate liposome.
[0029] The preparation method of the tamoxifen citrate liposome provided by the invention first dissolves tamoxifen citrate, phospholipid and cholesterol in a solvent.
[0030] According to the present invention, the phospholipid is preferably soybean lecithin.
[0031] The present invention does not limit the sources of tamoxifen citrate, phospholipids and cholesterol, which can be purchased co...
Embodiment 1
[0071] A tamoxifen citrate liposome.
[0072] Recipe: Formula No. 1 is used.
[0073] Preparation:
[0074] (1) Weigh soybean lecithin, cholesterol and tamoxifen citrate according to the formula dosage and add them to an eggplant-shaped bottle, add a mixture of methylene chloride and methanol with a volume ratio of 2:1, soybean lecithin and the added dichloromethane The ratio of the methyl chloride-methanol mixture is 8mg:6ml. After ultrasonic dissolution, the organic solvent is removed by gradient vacuum evaporation. The gradient vacuum evaporation process is as follows: -0.01Mpa for 30min; -0.04Mpa for 30min; -0.06Mpa for 20min; -0.1Mpa for 3min. The temperature of the evaporation process is 39°C, and the rotation speed is 85 / min. After the evaporation is complete, blow nitrogen gas for about 5-10 minutes to remove the residual organic solvent. A lipid film is obtained.
[0075] (2) Add ultrapure water with a ratio of 1ml to 4mg of soybean lecithin to 68°C, slowly add t...
Embodiment 2
[0078] A tamoxifen citrate liposome.
[0079] Formula: No. 2 formula is adopted.
[0080] Preparation:
[0081] (1) Weigh soybean lecithin, cholesterol and tamoxifen citrate according to the formula dosage and add them to an eggplant-shaped bottle, add a mixture of methylene chloride and methanol with a volume ratio of 1.8:1, soybean lecithin and the added dichloromethane The ratio of methyl chloride to methanol mixture is 8mg:8ml. After ultrasonic dissolution, the organic solvent is removed by gradient vacuum evaporation. The gradient vacuum evaporation process is as follows: -0.02Mpa for 40min; -0.05Mpa for 25min; -0.065Mpa for 15min; -0.09Mpa for 4min. The temperature of the evaporation process is 40°C, and the rotation speed is 88r / min. After the evaporation is complete, blow nitrogen gas for about 5-10 minutes to remove the residual organic solvent. A lipid film is obtained.
[0082] (2) Add ultrapure water with a ratio of 1ml to 6mg of soybean lecithin to 65°C, slow...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com