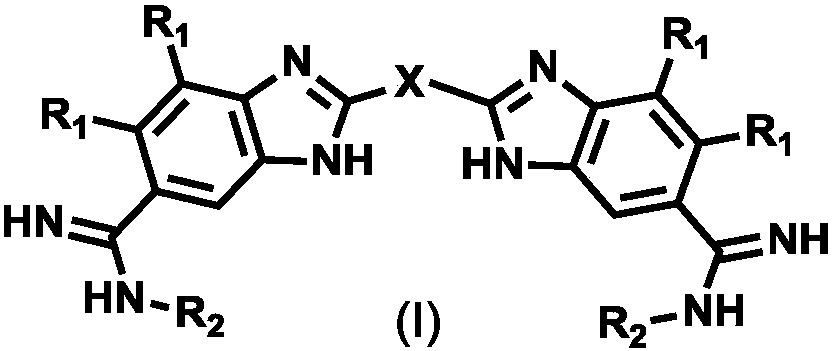
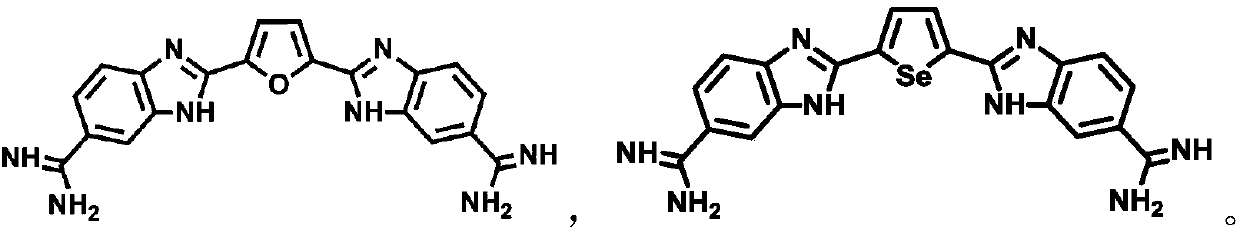
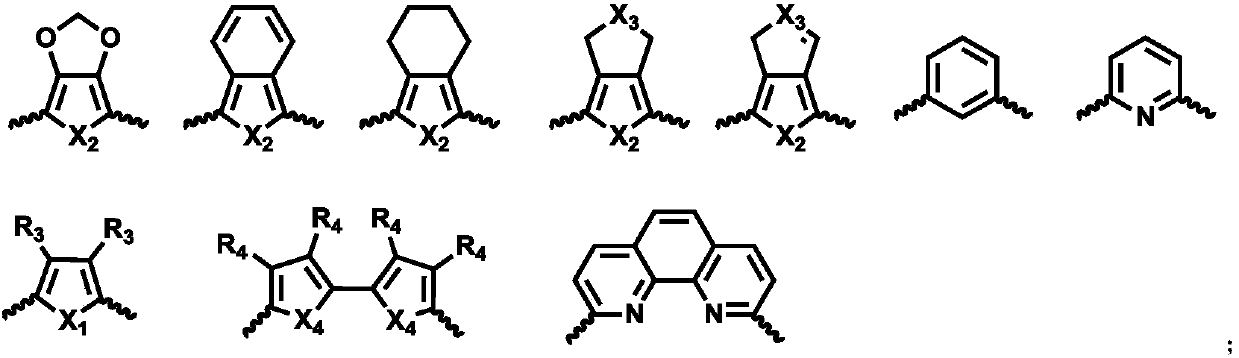
Five-membered six-membered heterocyclic compound as well as preparation method and application thereof
A compound and heterocyclic group technology, applied in the field of five-membered six-membered heterocyclic compounds and their preparation, can solve problems such as treatment failure, poor overall prognosis, and central nervous system infiltration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] The synthesis of embodiment 1, DB1976
[0113] DB1976 is prepared by the following synthetic route:
[0114]
[0115] Synthesis of Compound 2:
[0116] 4-Amino-3-nitro-benzamidine hydrochloride 1 (108.3 mg, 0.50 mmol) was dissolved in 10.7 mL of absolute ethanol, followed by the addition of 10% palladium on carbon (10.8 mg). After the system was completely replaced with hydrogen, the mixture was stirred at room temperature for 24 hours. Then the palladium carbon was removed by filtration, and the filtrate was spin-dried under vacuum to obtain yellow compound 2 (98.6 mg, quantitative reaction).
[0117] 1 H NMR (400MHz, CD 3 OD) δ7.11(dd, J=2.4,8.4Hz,1H),7.06(d,J=2.4Hz,1H),6.73(d,J=8.4Hz,1H); 13 C NMR (100MHz, CD 3 OD) δ 167.7, 143.9, 135.3, 121.3, 116.3, 115.4, 115.2; HRMS (ESI) m / z: calculated value of C 25 h 26 N 3 o 8 [M+NH 4 ] + = 496.1714, found value = 496.1715.
[0118] Synthesis of Compound 4:
[0119]Dissolve selenophene 3 (670.0mg, 5.10mmol) a...
Embodiment 2
[0126] Embodiment 2: the synthesis of compound 8
[0127] The preparation of compound 2 was the same as in Example 1.
[0128]
[0129] Synthesis of compound 7:
[0130] Dissolve 2,2-diselenol 6 (130.0mg, 5mmol) and tetramethylethylenediamine (711.0mg, 6.12mmol) in 1.6mL of anhydrous n-hexane, and completely replace the system with argon, then Argon gas was passed into the reaction solution for 6 minutes, and the reaction system was cooled to minus 4°C with an ice-salt bath, and the n-hexane solution of tert-butyllithium (8.5 mL, 1.3M) was slowly added to the reaction system dropwise, and the addition was completed. Afterwards, the reaction system was slowly warmed up to room temperature and refluxed for 30 minutes. 6.5 mL of freshly distilled THF was added, and the temperature of the reaction system was lowered to minus 40°C, and anhydrous N,N-dimethylformamide (1.49 g, 20.4 mmol) was slowly added dropwise into the reaction system.
[0131] After the addition, the react...
Embodiment 3
[0135] The compounds in Table 1 were prepared according to the same method as in Example 1. Wherein, for preparing compound 2 from compound 1, the non-hydrochloride salt structure of compound 1 is adopted:
[0136]
[0137] Other preparation conditions are with embodiment 1.
[0138] Table 1: Specific compounds and their mass spectral data
[0139]
[0140]
[0141]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com