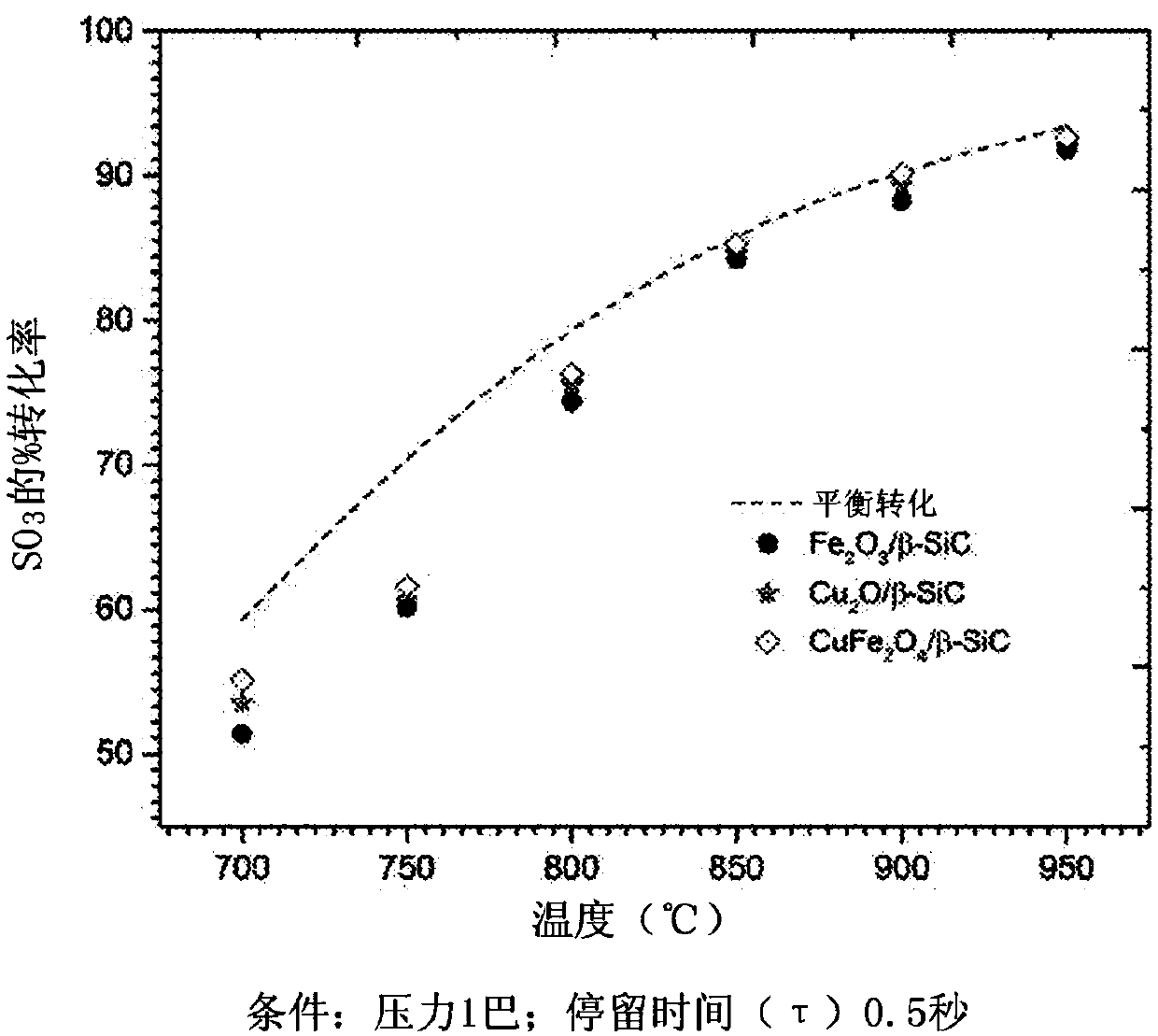
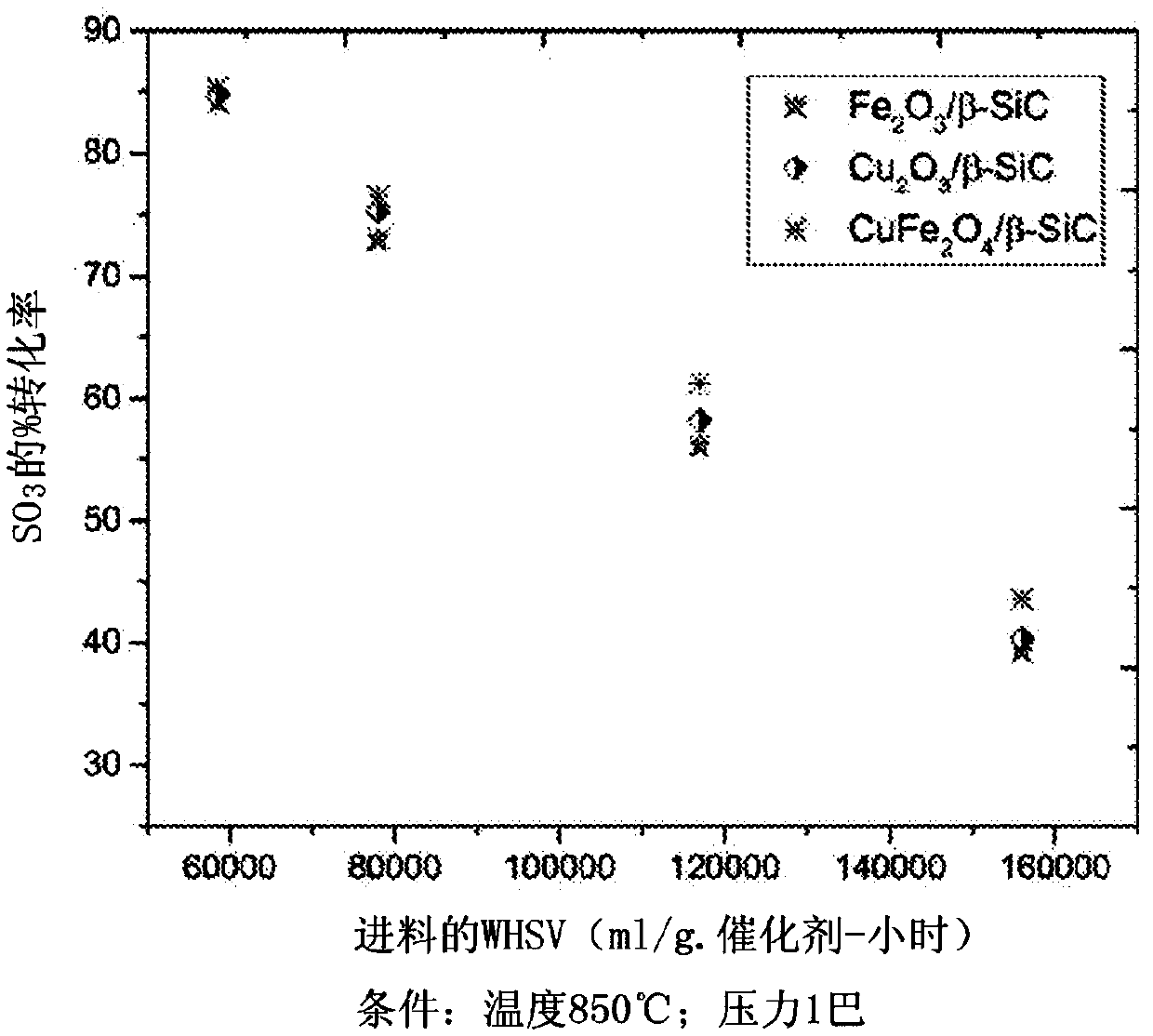
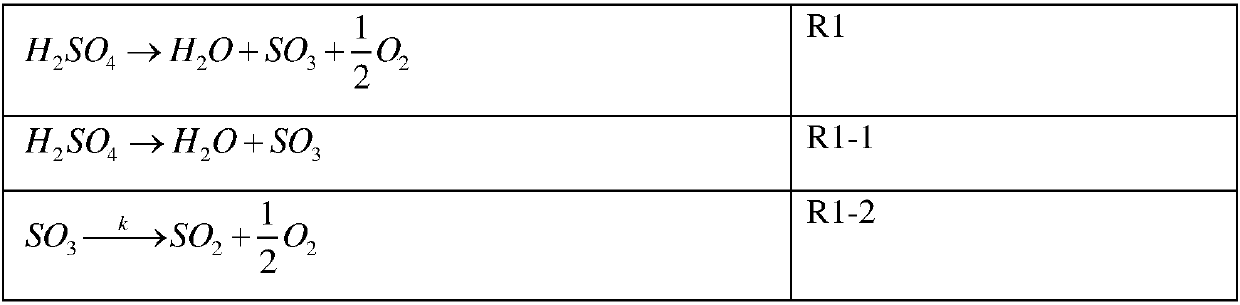
Process for conversion of sulfur trioxide and hydrogen production
A sulfur trioxide and sulfur dioxide technology, applied in chemical instruments and methods, hydrogen/synthesis gas production, oxygen/ozone/oxide/hydroxide, etc., can solve problems such as low production and high operating costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Pretreatment of Catalyst Supports
[0083] The catalyst support is obtained by using a synthetic method called Pretreatment Method (PTM). Silicon carbide (β-SiC) extrusions (diameter 2 mm) were supplied by SICAT Sarl (France) and are hereafter referred to as β-SiC(R) or β-SiC. Etch β-SiC(R) samples with a 1:1 HF solution in water under ultrasound for 3 min to 5 min at room temperature to remove S from the surface of β-SiC i o x C y / S i o z . The samples were filtered and washed with plenty of deionized water until the filtrate pH reached 6.5 to 7, then the samples were dried under vacuum at 120 °C for 3 hours to 5 hours, hereafter referred to as β-SiC(P) or simply free of β-SiC of silicon dioxide. The dried sample (β-SiC(P)) was subsequently oxidized in atmospheric air at between 700°C and 1000°C for a period of 2 hours to 6 hours to obtain pretreated β-SiC or simply β- SiC(PT).
[0084] Example 1(b)
[0085] Catalyst Fe 2 o 3 / Preparation of β-SiC(R) (for...
Embodiment 2
[0094] Catalyst Cu 2 Preparation of O / β-SiC(R) (for comparison)
[0095] 1.8741g copper precursor (Cu(NO 3 ) 2 .3H 2 O) Dissolved in 10 ml of distilled water, then added 10 g of pre-dried and degassed 2 mm size β-SiC(R) extrudates. Then, the resulting mixture was sonicated for about 30 minutes, so that all β-SiC(R) was fully immersed in the solution. After half an hour, β-SiC(R) was separated from the solution and dried at 80° C. for 30 minutes, then added again to the remaining solution, so that the entire copper solution was absorbed by β-SiC(R). Finally, the impregnated substrates were air dried at 100 °C for 1 h and then calcined at 500 °C for 2 h. The final catalyst is 5% Cu supported on β-SiC(R) 2 O. 2% to 15% (w / w) supported copper(I) oxide catalysts were also prepared by a similar method.
[0096] Example 2(b)
[0097] Catalyst Cu 2 Preparation of O / β-SiC(PT) (for comparison)
[0098] 5% Cu was prepared using the same protocol as used in Example 1(b) 2 O / β-...
Embodiment 3
[0100] Catalyst Cr 2 o 3 / Preparation of β-SiC(R) (for comparison)
[0101] 1.101g ammonium chromate (Cu(NO 3 ) 2 .3H 2 O) Dissolved in 10 ml of distilled water, then added 10 g of pre-dried and degassed 2 mm size β-SiC(R) extrudates. Then, the resulting mixture was sonicated for about 30 minutes, so that all β-SiC(R) was fully immersed in the solution. After half an hour, β-SiC(R) was separated from the solution and dried at 80° C. for 30 minutes, then added again to the remaining solution, so that the entire ammonium chromate solution was absorbed by β-SiC(R). Finally, the impregnated substrates were air dried at 100 °C for 1 h and then calcined at 500 °C for 2 h. The final catalyst is 5% Cr supported on β-SiC(R) 2 o 3 . 2% to 15% (w / w) supported chromium(III) oxide catalysts on β-SiC(R) supports were also prepared by a similar method.
[0102] Example 3(b)
[0103] Catalyst Cr 2 o 3 / Preparation of β-SiC(PT) (for comparison)
[0104] 5% Cr was prepared using...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com