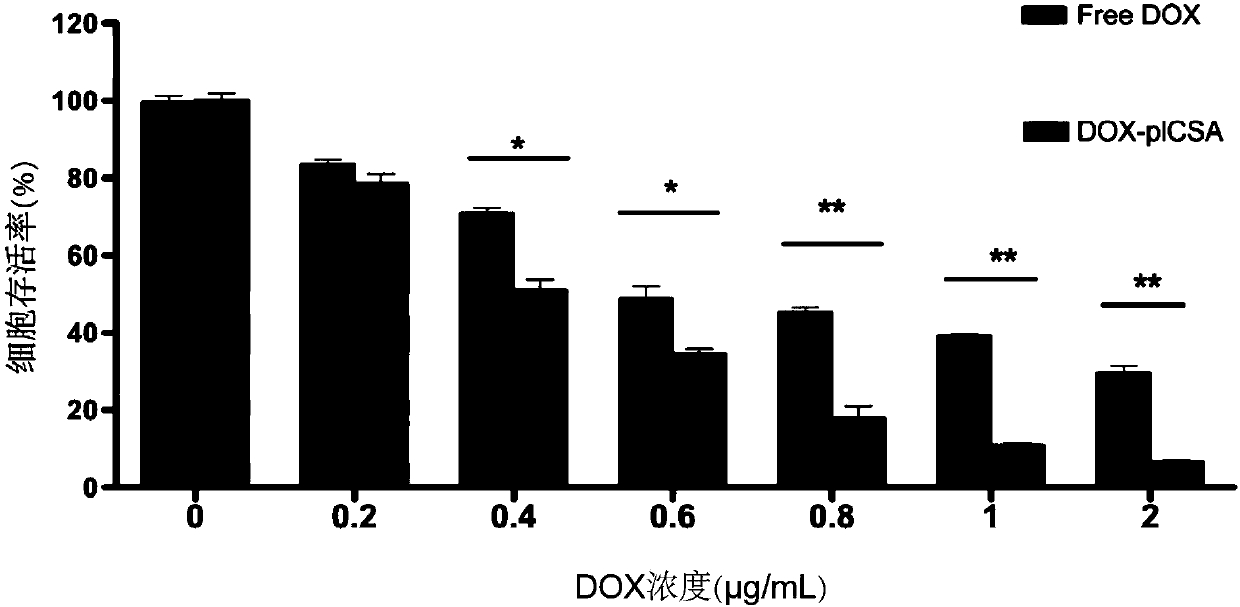
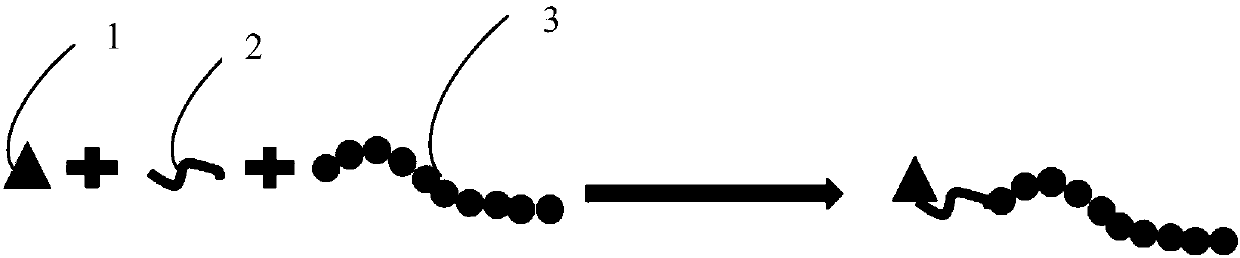Peptide-drug conjugate of target placenta-like chondroitin sulfate A, and preparation method and application of peptide-drug conjugate
A technology of chondroitin sulfate and drug conjugates, which is applied in the field of medicine, can solve the problems of no receptor delivery system reported in the literature, achieve the effects of prolonging the circulation time in the body, simple and easy preparation method, and avoiding side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
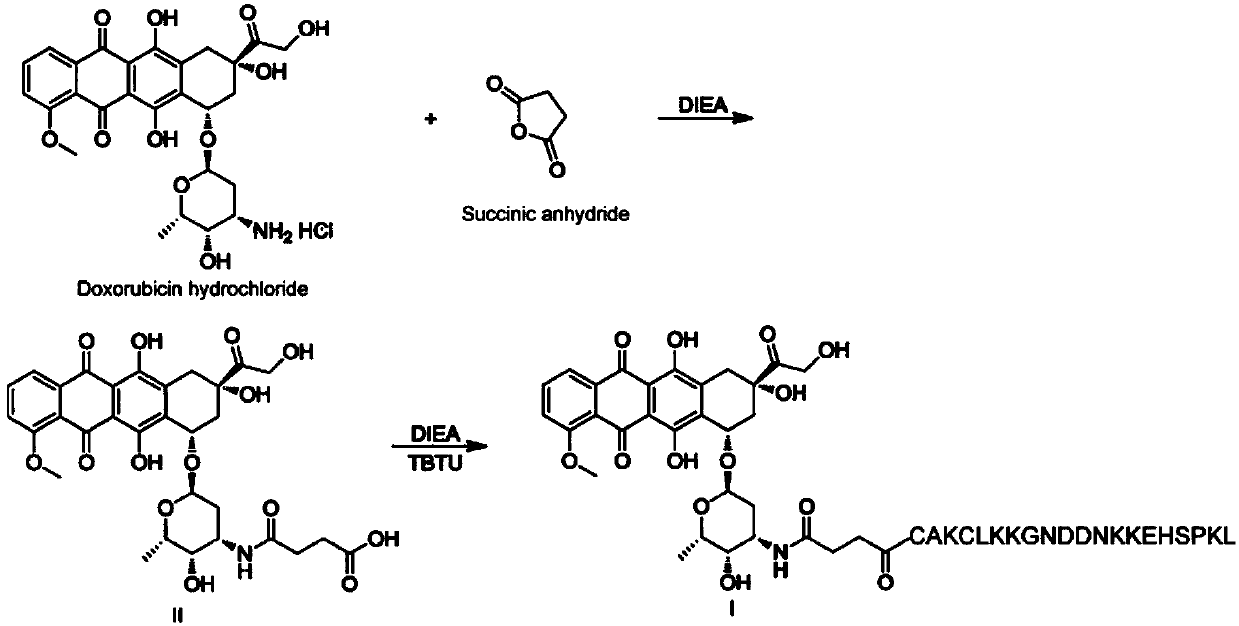
[0060] Preparation of a polypeptide-drug conjugate targeting placenta-like chondroitin sulfate A (pl-CSA): first react doxorubicin hydrochloride with succinic anhydride to introduce a carboxyl group on the doxorubicin molecule; then carboxylate The doxorubicin and the polypeptide undergo amide reaction, so that the terminal amino group of the polypeptide fragment reacts with the carboxyl group on the carboxylated doxorubicin to generate a targeted polypeptide-drug conjugate. The reaction equation is as follows:
[0061]
[0062] Specifically, the following steps are included:
[0063] (1) Synthesis of Compound II
[0064] Add 1.0g (1.72mmol) doxorubicin hydrochloride, 0.52g (5.2mmol) succinic anhydride, 30mL tetrahydrofuran (THF) to the 50mL reaction flask, add dropwise 0.66g (5.12mmol) of N,N-diisopropylethyl Amine (DIEA), stirred at 27°C for 6 hours, evaporated to dryness to obtain a waxy substance, added water and stirred, a yellow solid precipitated, suction filtered,...
Embodiment 2
[0068] A preparation of a polypeptide-drug conjugate targeting pl-CSA. Firstly, paclitaxel is reacted with succinic anhydride to introduce a carboxyl group on the paclitaxel molecule; The amino group reacts with the carboxyl group on the carboxylated paclitaxel to generate a polypeptide-drug conjugate. The reaction equation is as follows:
[0069]
[0070] Specifically, its preparation process includes the following steps:
[0071] (1) Synthesis of compound III
[0072]Add 1.0g (1.72mmol) paclitaxel, 0.52g (5.2mmol) succinic anhydride, 30mL tetrahydrofuran (THF) to the 50mL reaction bottle, add dropwise 0.66g (5.12mmol) N, N-diisopropylethylamine (DIEA) , Stir at 27°C for 6h, evaporate the solvent to dryness to obtain a waxy substance, add water and stir, a yellow solid precipitates, filter with suction, wash twice with water, and dry to obtain 1.15g of a yellow solid.
[0073] (2) Synthesis of Compound IV
[0074] Add 0.22g (0.32mmol) compound III, 0.10g (0.31mmol) O-b...
Embodiment 3
[0076] A method for preparing a polypeptide-drug conjugate targeting pl-CSA, the difference from Example 1 is that this example uses 0.1 g of a polypeptide such as SEQUENCE NO.2 (EDVKDINFDTKEKFLAGCLIVSFHEGKC) to replace the one in Example 1 Peptides used.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com