Method for detecting nitrofuran antibiotics or quinolone antibiotics in drinking water
A nitrofuran and quinolone technology, applied in measurement devices, instruments, fluorescence/phosphorescence, etc., can solve the problems of unstable excitation wavelength dependence, cumbersome processing, and high operating costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
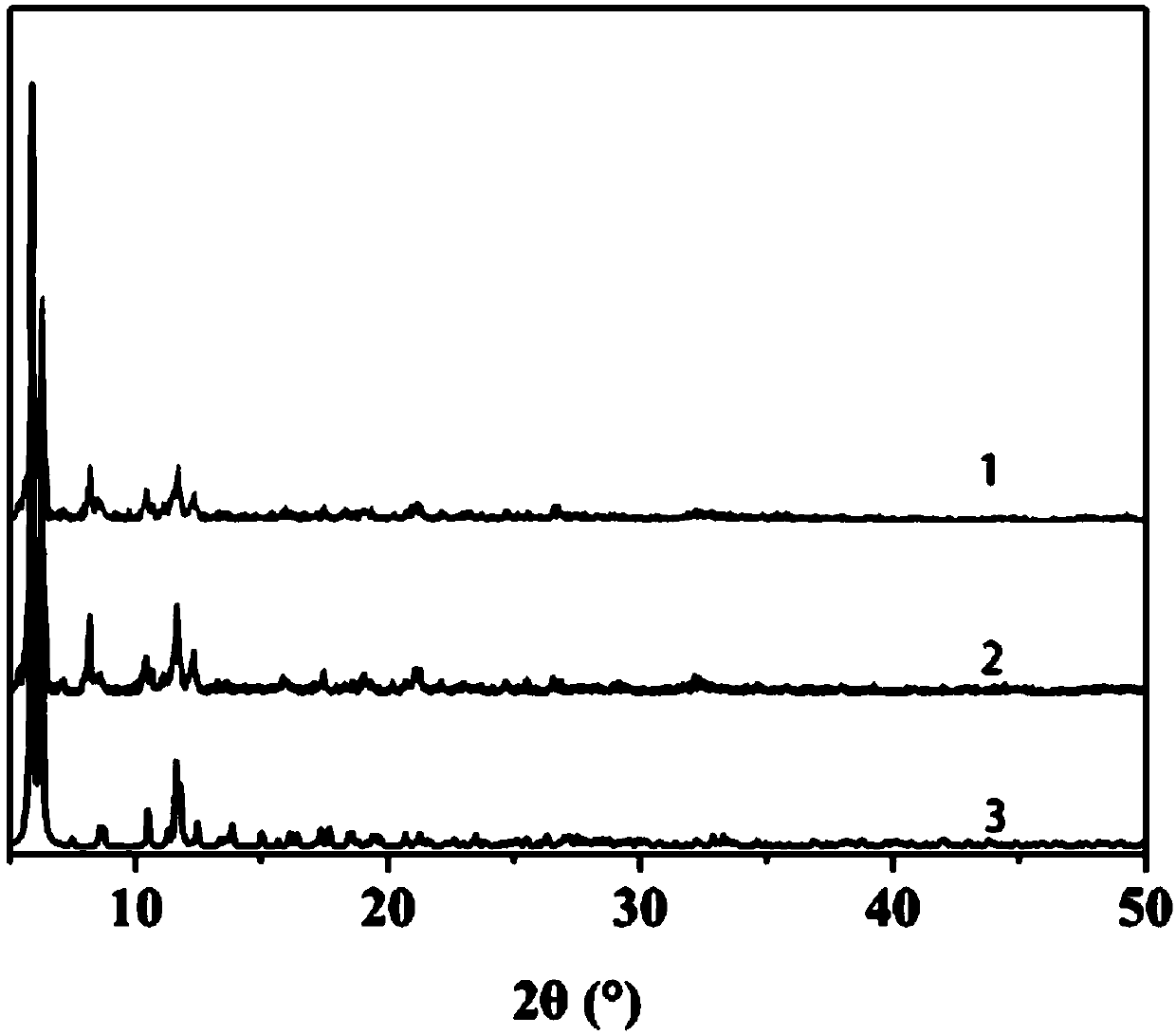
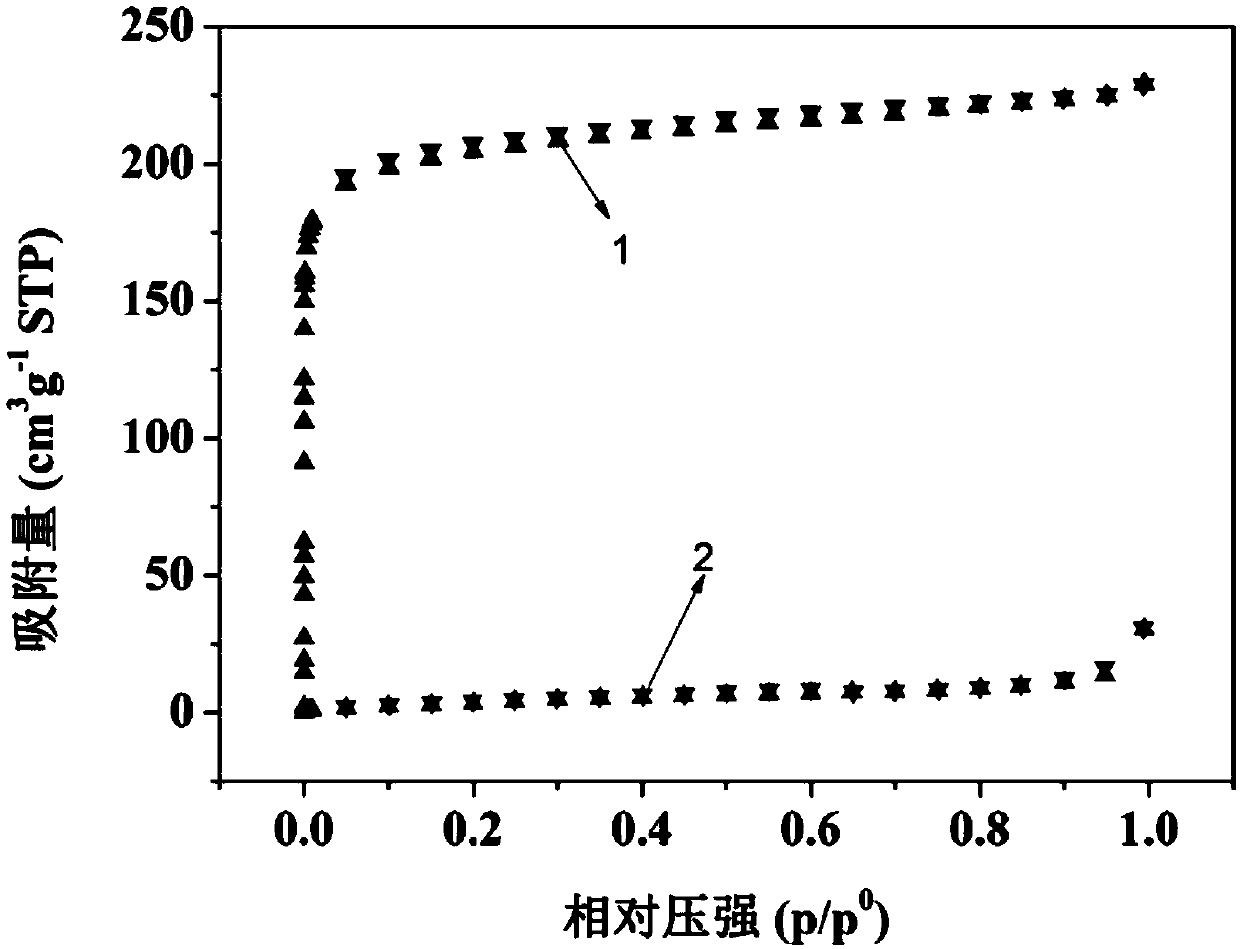
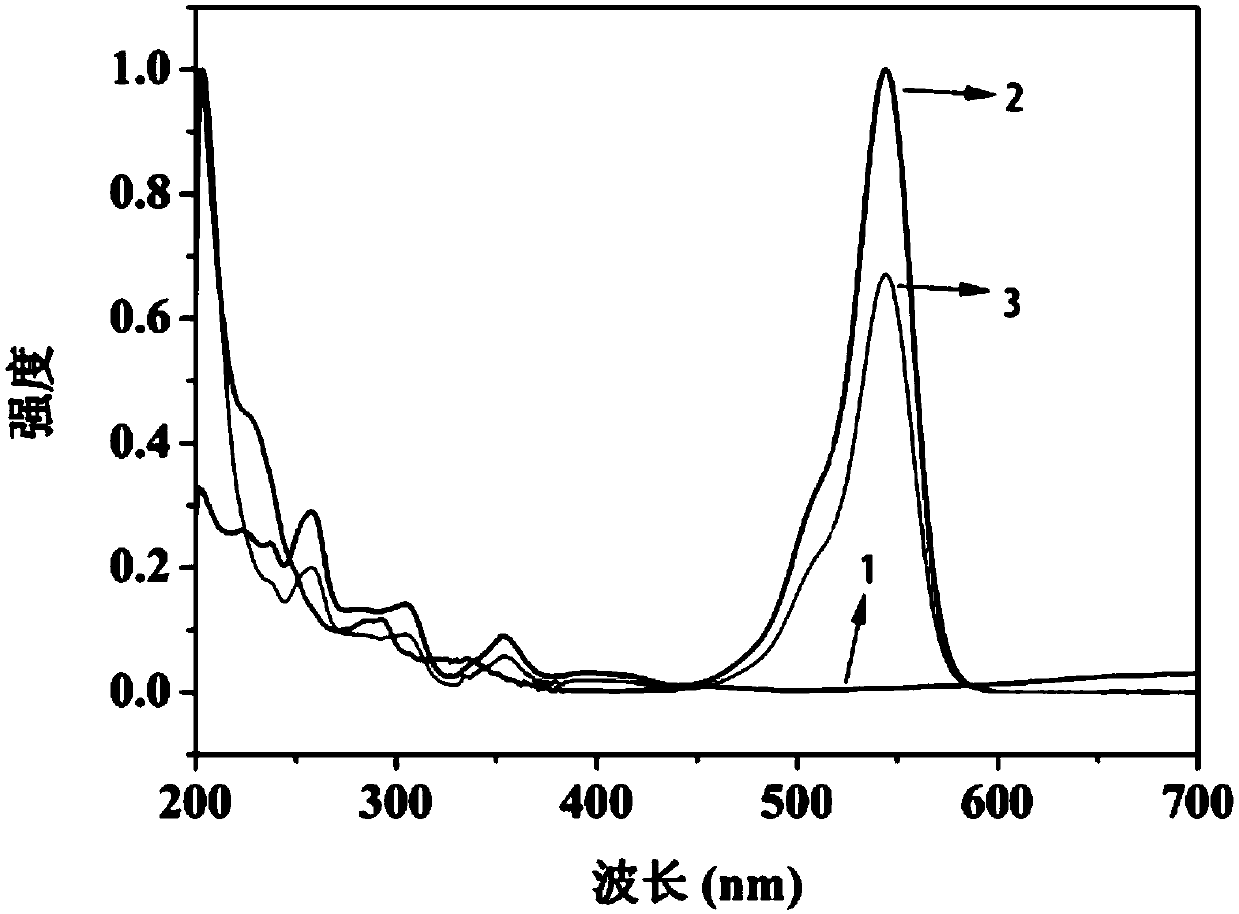
Image
Examples
specific Embodiment approach 1
[0058] Specific embodiment one: a method for detecting nitrofuran antibiotics or quinolone antibiotics in drinking water in this embodiment is carried out according to the following steps:
[0059] 1. Preparation of Tb-dcpcpt crystals:
[0060] ①, Tb(NO 3 ) 3 ·6H 2 O, 3-(3,5-dicarboxyphenyl)-5-(4-carboxyphenyl)-1-hydrogen-1,2,4-triazole, N,N'-dimethylformamide and h 2 O mixed to obtain a mixture, the mixture is placed in a high-temperature reactor, under the condition of a temperature of 150°C to 200°C, sealed for 24h to 72h for solvothermal reaction, and a crystal sample is obtained;
[0061] The 3-(3,5-dicarboxyphenyl)-5-(4-carboxyphenyl)-1-hydrogen-1,2,4-triazole and Tb(NO 3 ) 3 ·6H 2 The molar ratio of O is 1:(0.5~2); the 3-(3,5-dicarboxyphenyl)-5-(4-carboxyphenyl)-1-hydrogen-1,2,4-tri The molar ratio of nitrogen azole to N,N'-dimethylformamide is 0.1mmol:(5-10)mL; the N,N'-dimethylformamide and H 2 The volume ratio of O is 1:(0.1~2);
[0062]②. Wash the crystal ...
specific Embodiment approach 2
[0077] Specific embodiment 2: The difference between this embodiment and specific embodiment 1 is: 3-(3,5-dicarboxyphenyl)-5-(4-carboxyphenyl)-1-hydrogen described in step ① -1,2,4-Triazole and Tb(NO 3 ) 3 ·6H 2 The molar ratio of O is 1:(0.5~1). Others are the same as in the first embodiment.
specific Embodiment approach 3
[0078] Specific embodiment three: the difference between this embodiment and specific embodiment one or two is: 3-(3,5-dicarboxyphenyl)-5-(4-carboxyphenyl) described in step 1. -The volume ratio of the mole of 1-hydrogen-1,2,4-triazole to N,N'-dimethylformamide is 0.1mmol:(8~10)mL. Others are the same as those in the first or second embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com