Herpes zoster virus vaccine, and preparation method and applications thereof
A herpes zoster virus and vaccine technology, applied to biochemical equipment and methods, viruses, antiviral agents, etc., can solve problems that do not involve adjuvant technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 6
[0163] Example 6: Identification of antigenic sites of proteins expressed by herpes zoster virus gene 68
[0164] Partially overlapping 13-peptide or 20-peptide fragments covering the full amino acid sequence were synthesized for gene 68. Using these fragments, we cultured and stimulated 24 polyclonal CD4 cell lines cultured from pre-vaccination and 14 days post-vaccination polyclonal CD4 cell lines for 24 h, and then measured the concentration of interferon-γ in the supernatant. The sites that resulted in T cell responses to each protein were thus identified.
[0165] The protein expressed by gene 68 is gE protein. The results demonstrated that the major CD4 T cell loci were in the amino acid sequence 190-209 and 280-299. 75% of the vaccinated T cells responded positively to these two loci. Additional loci to which at least one third of the vaccinated T cells responded included amino acid sequences 46-65, 154-173, 199-219, 334-353 and 415-434. In addition, one-third of the...
Embodiment 7
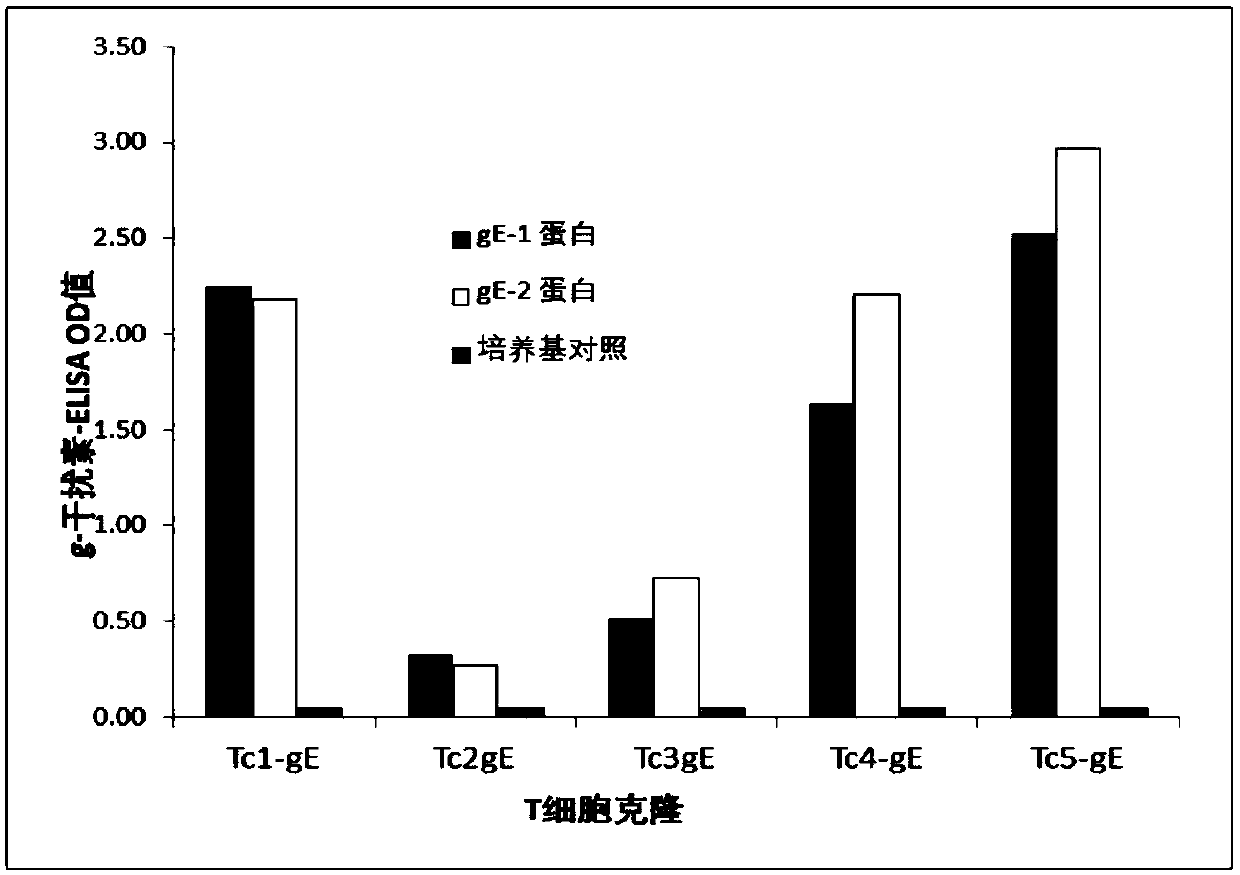
[0169] Example 7: Isolation and Preparation of T Cell Clones of the Main T Cell Locus of the gE Protein
[0170] The two major T-cell loci of the gE protein are amino acid sequence 190-210 (RIYGVRYTETWSFLPSLTCT) and amino acid sequence 280-299 (EIEPGVLKVLRTEKQYLGVY). We cloned T cells targeting these two sites from the blood leukocytes of two vaccinated individuals and expanded them in culture.
[0171]Firstly, the quadruplets of the above two polypeptides were artificially synthesized and labeled with Allophycocyanin. The labeled quadruplets were used to react with the isolated blood leukocytes of the vaccinators. Allophycocyanin-labeled CD4-positive cells were isolated by flow cytometry. Put the isolated cells into a 96-well cell culture plate (1 cell per well) with γ-ray irradiated whole blood leukocytes (200,000 cells per well) and phytohemagglutinin (1.6 μg / well empty) To cultivate. And add human IL-2 (32 U per ml) the next day. Cultured continuously for 14 days. Fr...
Embodiment 8
[0174] Embodiment 8: the stable cell line CHO construction of the expressed gE protein
[0175] The gE protein is a glycosylated protein that needs to be expressed in eukaryotic cells to obtain a more realistic protein structure. Therefore, we constructed two Chinese Hamster Ovarian (CHO) cell lines expressing different gE protein sequences. The first cell line expresses the gE extra-enveloped sequence (amino acid sequence 31-538) without the signal peptide. The second CHO cell line expresses the fusion protein of gE protein sequence outside the envelope (amino acid sequence 31-538) and inside the envelope (amino acid sequence 560-623). The protein expressed by the second CHO cell line does not contain a transmembrane sequence (amino acid sequence 539-559) because the product of the transmembrane sequence has poor solubility and is easy to precipitate.
[0176] The construction of the cell line was carried out according to the conventional method. Primers were designed base...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com