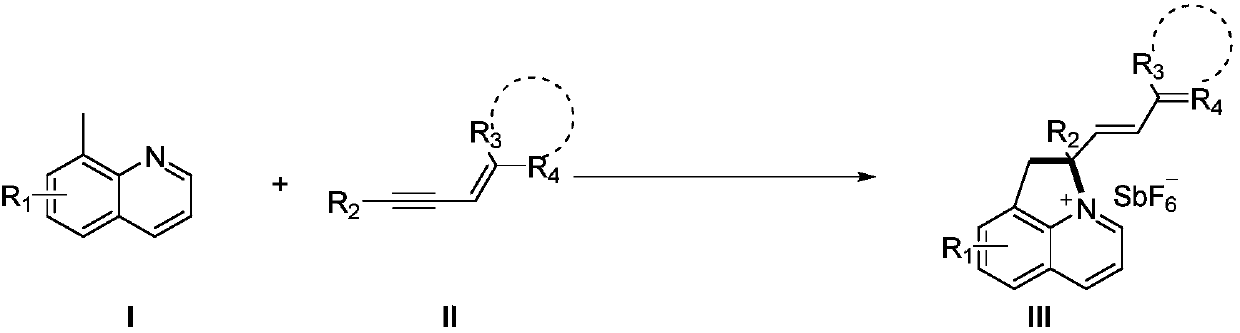
Synthesis method of nitrogenous heterocyclic quaternary salt compounds
A synthesis method and compound technology, which is applied in the field of synthesis of nitrogen-containing heterocyclic quaternary salt compounds, and can solve problems such as difficult access to quaternary ammonium salts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
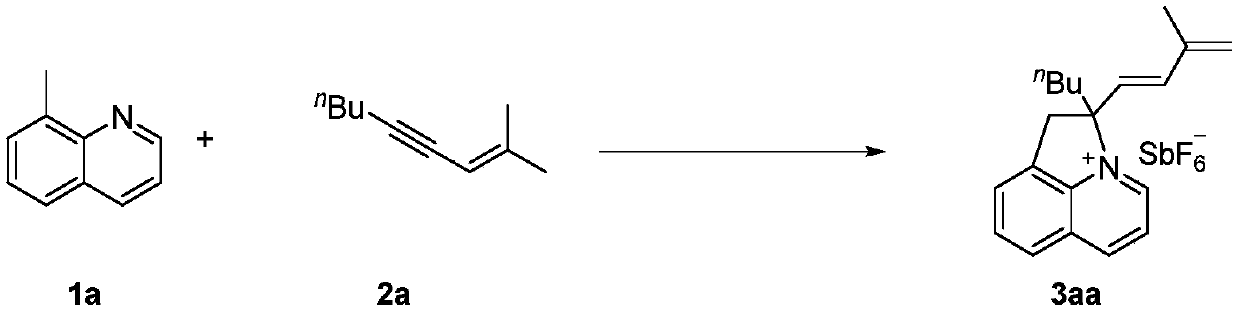
Embodiment 1
[0030]
[0031] Above-mentioned reaction condition optimization experiment is shown in the following table:
[0032]
[0033]
[0034] Reaction conditions: 1a (0.2mmol), 2a (0.3mmol), [Cp*Rh(OAc) 2 ] (8mol%), organic solvent (2.0mL), additives and oxidizing agents. Wherein the label 3 adopts 2a as 0.2 mmol.
[0035] Under the protection of inert gas, add [Cp*Rh(OAc) 2 ] (8mol%), AgSbF 6 (0.2mmol), AgOAc (0.3mmol) and DCE (2.0mL), stirred for ten minutes under backlight conditions, then added compound 1a (0.2mmol) and compound 2a (0.3mmol), placed in 100 ° C oil bath for reaction, in After the reaction was complete (24 hours), the sealed tube was removed from the oil bath and cooled to ambient temperature. The reaction was filtered through celite, eluted with DCM:MeOH=10:1 and concentrated. The crude product was then transferred to a tube with a magnetic stir bar, then DCM (2.0 mL), water (2.0 mL) and NaSbF were added at room temperature 6 (100mg). After stirrin...
Embodiment 2
[0038]
[0039] Under the protection of inert gas, add [Cp*Rh(OAc) 2 ] (8mol%), AgSbF 6(0.2mmol), AgOAc (0.3mmol) and DCE (2.0mL), stirred for ten minutes under the backlight condition, then added compound 1a (0.2mmol) and compound 2b (0.3mmol), placed in 100 ° C oil bath for reaction, in After the reaction was complete (24 hours), the sealed tube was removed from the oil bath and cooled to ambient temperature. The reaction was filtered through celite, eluted with DCM:MeOH=10:1 and concentrated. The crude product was then transferred to a tube with a magnetic stir bar, then DCM (2.0 mL), water (2.0 mL) and NaSbF were added at room temperature 6 (100mg). After stirring the reaction mixture for 10 minutes, the organic layer was separated and the aqueous layer was extracted twice with DCM. The organic layer was evaporated and purified by silica gel chromatography (DCM:MeOH=15:1) to give the product 3ab.
[0040] 3ab, 69.0mg, 62%. 1 H NMR (400MHz, CD 2 Cl 2 )δ10.19(d,J=...
Embodiment 3
[0042]
[0043] Under the protection of inert gas, add [Cp*Rh(OAc) 2 ] (8mol%), AgSbF 6 (0.2mmol), AgOAc (0.3mmol) and DCE (2.0mL), stirred for ten minutes under the backlight condition, then added compound 1a (0.2mmol) and compound 2c (0.3mmol), placed in 100 ° C oil bath for reaction, in After the reaction was complete (24 hours), the sealed tube was removed from the oil bath and cooled to ambient temperature. The reaction was filtered through celite, eluted with DCM:MeOH=10:1 and concentrated. The crude product was then transferred to a tube with a magnetic stir bar, then DCM (2.0 mL), water (2.0 mL) and NaSbF were added at room temperature 6 (100mg). After stirring the reaction mixture for 10 minutes, the organic layer was separated and the aqueous layer was extracted twice with DCM. The organic layer was evaporated and purified by silica gel chromatography (DCM:MeOH=15:1) to give the product 3ac.
[0044] 3ac, 82.0mg, 78%. 1 H NMR (400MHz, Acetone-d 6 )δ10.73(d,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com