Anticoagulated small-molecule compound, application thereof and drug including compound
A small molecular compound and small molecule technology, applied in the field of biomedicine, can solve the problems of narrow drug treatment window, easy to cause bleeding complications, etc., achieve the effect of small bleeding side effects, solve bleeding side complications, and prolong thromboplastin time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The synthetic equation of the anticoagulant small molecular compound that selectively inhibits blood coagulation factor XIa is as follows:
[0039]
[0040] Will with CH 2 (COOH) 2 According to the molar ratio of 1:1.1, the reaction is carried out at 76 degrees Celsius to obtain then With ethanol according to the molar ratio of 1:1, react at 6 degrees Celsius to get
[0041] 2) Will According to the molar ratio of 1:22 with thionyl chloride, react under the condition of 79 degrees Celsius to obtain
[0042] 3) The obtained in step 1) and obtained in step 2) According to the molar ratio of 1:1, the reaction is carried out at 0 degrees Celsius to obtain
Embodiment 2
[0044] Anticoagulant activity of the anticoagulant small molecular compound provided by the invention
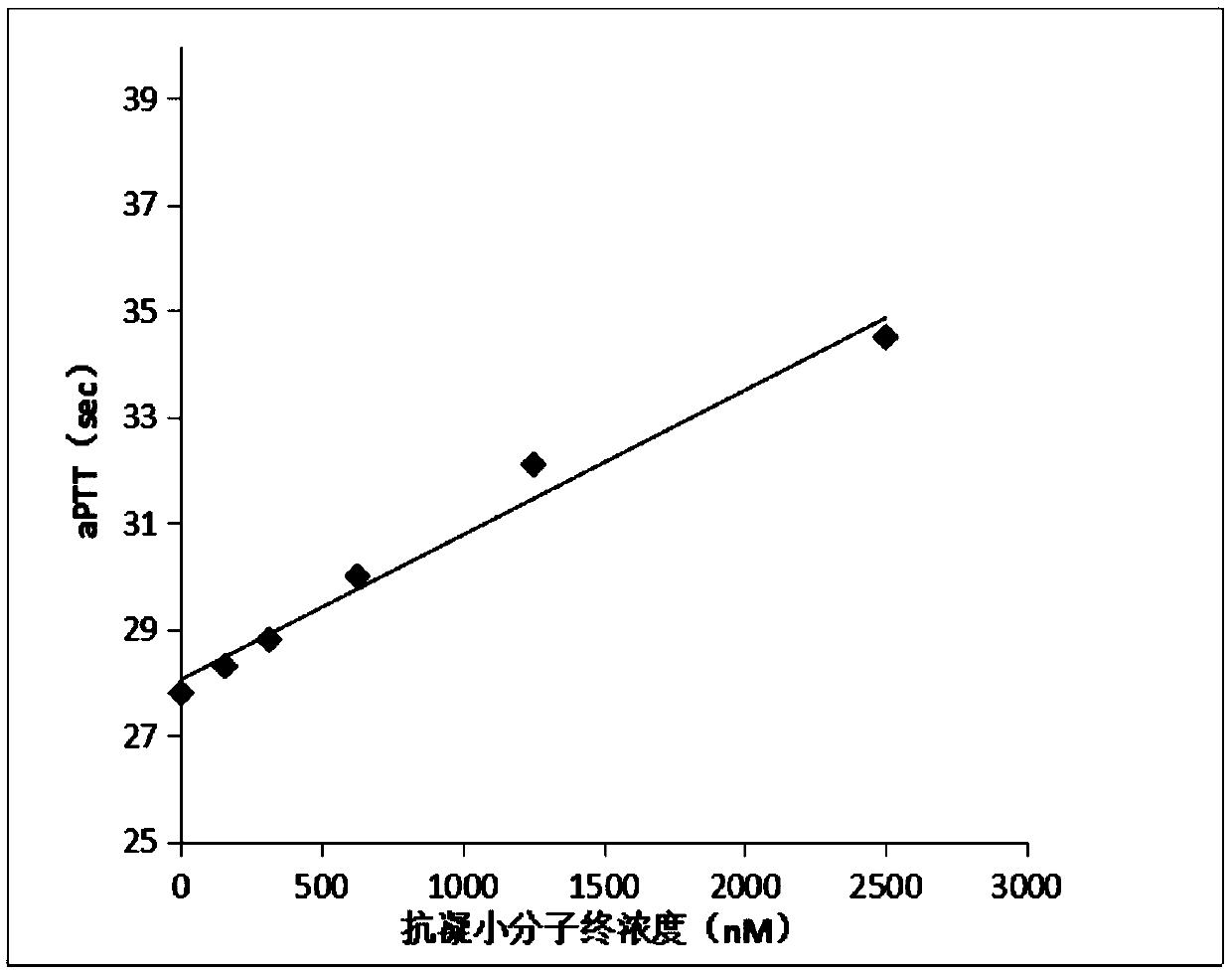
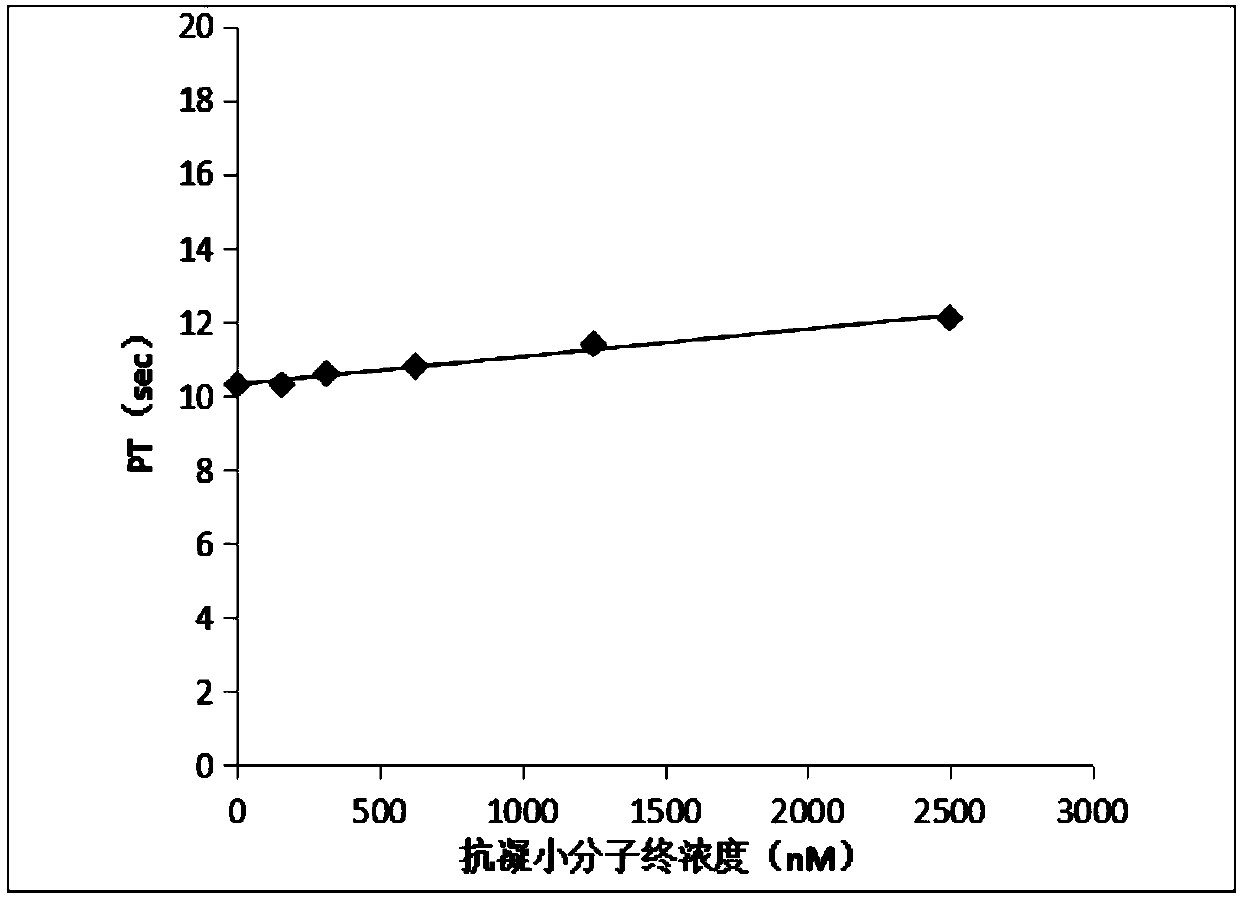
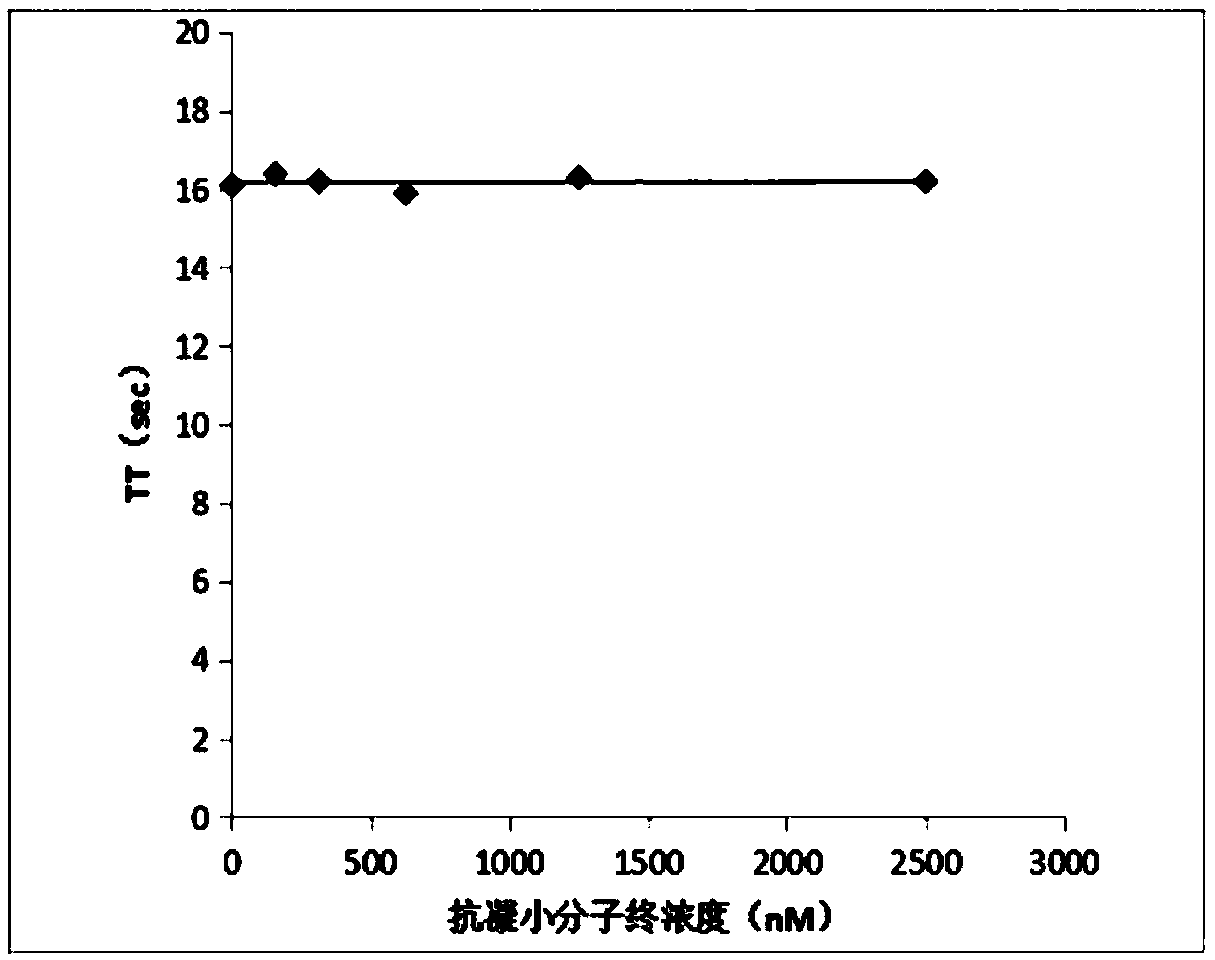
[0045] The anticoagulant activity of the anticoagulant small molecular compound provided by the invention is observed by detecting activated partial thromboplastin time (aPTT) and prothrombin time (PT).
[0046] Determination of prothrombin time (PT): Prepare 1 part of anticoagulant small molecule compound with different concentrations and 4 parts of normal human plasma, mix well and incubate at 37°C for 15 minutes, then use a coagulation instrument S5100 (SYSMEX, Japan), and set the measurement parameters as follows: mix Plasma 50ul+50ul original aPTT reagent+50ul original calcium chloride reagent. The instrument reads the result. Each concentration was repeated twice, and the average value was taken. Experimental results such as figure 1 shown.
[0047] Determination of prothrombin time (PT): Prepare 1 part of anticoagulant small molecule compound with different concen...
Embodiment 3
[0051] Effects of Anticoagulant Small Molecular Compounds on Coagulation Time in Rats
[0052] 40 SPF grade SD rats (provided by the Experimental Animal Center of Guangdong Medical University), weighing 250 ± 50g, were randomly divided into 4 groups, namely blank control group (distilled water), low-dose anticoagulant small molecule group (20mg / kg), Anticoagulant small molecule medium dose group (40mg / kg) and anticoagulant small molecule high dose group (80mg / kg), 10 rats in each group. Rats were intragastrically administered, and after 3 hours, blood was taken from the tail vein or eyeballs, injected into a plastic tube added with 3.8% sodium citrate (blood: anticoagulant = 9:1), and shaken gently. Centrifuge at 1500Xg for 10min to separate the plasma. Determination of aPTT and PT, the measurement method is as described in (2). The results are shown in Table 1
[0053] the result shows
[0054] Anticoagulant small molecule compounds can significantly prolong plasma activa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com