Molecular fluorophores and preparation method thereof and use for short wavelength infrared imaging
An intermolecular, electron-accepting technology, applied in pharmaceutical formulations, preparations for in vivo experiments, organic chemistry, etc., can solve the problems of large size and low real-time imaging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0191] Example 1: Synthesis of IRETBN-PEG1700 and IRETBN-PEG600
[0192]
[0193] (1) 5-(5-(2,6-bis((6-bromohexyl)oxy)phenyl)thiophen-2-yl)-2,3-dihydro-thieno[3,4-b] Synthesis of [1,4]dioxine (compound 2):
[0194] Under Ar atmosphere, tributyl(2,3-dihydrothieno[3,4-b][1,4]dioxin-5-yl)stannane (860mg, 2.0mmol) and To a solution of compound 1 (720mg, 1.2mmol) in 10mL of toluene, Pd(PPh 3 ) 4 (71 mg, 0.061 mol). The mixture was stirred at 110 °C for 24 h. After cooling to room temperature, the mixture was poured into water and extracted twice with ethyl acetate, and the organic phase was washed with MgSO 4 Dry and evaporate in vacuo. The crude product was subjected to column chromatography on silica gel with PE / DCM 1:1 to afford compound 2 as a pale yellow oil (590 mg, 45%).
[0195] 1 H NMR (400MHz, CDCl 3 ): δ7.45(d, J=3.9Hz, 1H), 7.24(d, J=3.9Hz, 1H), 7.17(t, J=8.3Hz, 1H), 6.61(d, J=8.4Hz, 2H ),6.21(s,1H),4.37–4.32(m,2H),4.28–4.23(m,2H),4.01(t,J=6.2Hz,4H),3.38(t,...
Embodiment 2
[0207] Example 2: Synthesis of IREF-PEG600 and IREFN-PEG600.
[0208]
[0209] (1) Synthesis of 5-(9H-fluoren-2-yl)-2,3-dihydrothieno[3,4-b][1,4]dioxine (compound 6):
[0210] Under a protective gas atmosphere, 2-bromo-9H-fluorene (compound 5) (5.0 g, 20.4 mmol) and tributyl (2,3-dihydrothieno[3,4-b][1,4] Dioxin-5-yl) stannane (9.2 g, 21.4 mmol) was dissolved in 40 mL of toluene, then Pd(PPh 3 ) 4 (200mg). After refluxing for 6 h, the crude product was subjected to column chromatography on silica gel to afford compound 6 as a pale yellow solid (5.8 g, 94%).
[0211] 1 H NMR (500MHz, chloroform-d) δ7.94 (dd, J = 3.8, 1.7Hz, 1H), 7.82–7.74 (m, 3H), 7.56 (dd, J = 7.3, 2.0Hz, 1H), 7.40 ( td,J=7.5,2.7Hz,1H),7.36–7.29(m,1H),6.41–6.26(m,1H),4.37–4.31(m,2H),4.29–4.23(m,2H),3.95( s, 2H). 13C NMR (126MHz, chloroform-d) δ37.60, 65.10, 65.41, 98.00, 118.68, 120.45, 120.62, 123.15, 125.43, 125.64, 127.22, 127.41, 132.37, 138.60, 140.84, 142.401, 40.1, 40.1
[0212] HRMS (ESI) fo...
Embodiment 3
[0226] Example 3: Synthesis of IREFNS
[0227]
[0228] Compound 8 (100 mg, 0.069 mmol) was dissolved in 10 mL THF and dimethylamine (2.0 M in THF, 2 mL), then stirred at 50 °C for 6 h. Afterwards, the solvent was evaporated in vacuo. The dark green solid and 1,2-oxathiolane 2,2-dioxide 122 mg (1 mmol) were dissolved in 5 mL THF, and the solution was stirred overnight. Afterwards, the solution was filtered, washed several times with acetone and ethyl acetate. IREFNS (110 mg) was obtained as a green solid.
[0229] HRMS (ESI) for C 88 h 120 N 8 o 16 S 8 , ([M+H + ]) The calculated value is 1800.6588, and the measured value is 1800.6545.
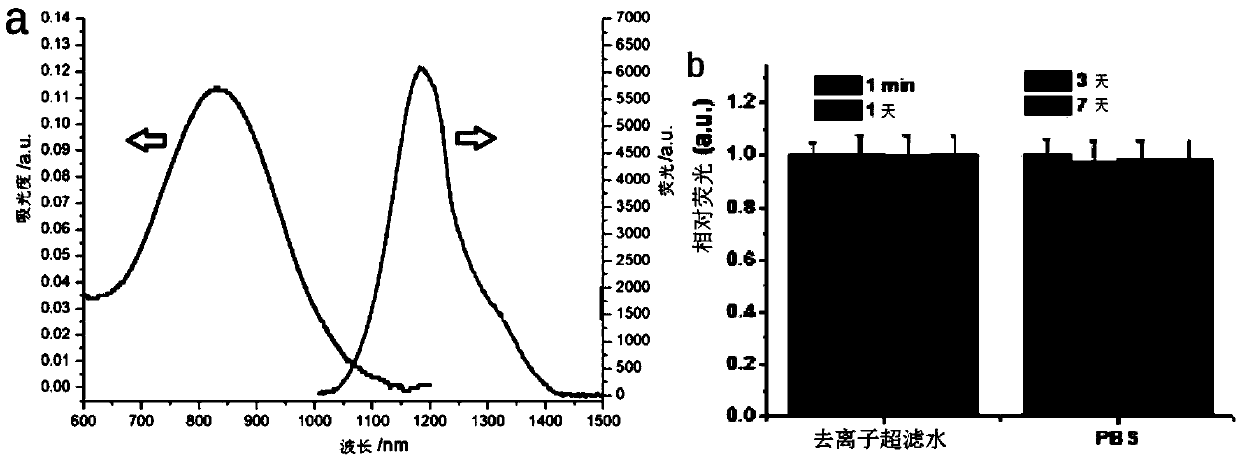
[0230] IREFNS in H 2 Optical parameters in O: absorption peak λ=795nm, emission peak λ em =1047nm, the absorption coefficient K=6.2L / g.cm at 808nm, the quantum yield is 0.46% (using 808nm excitation)
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com