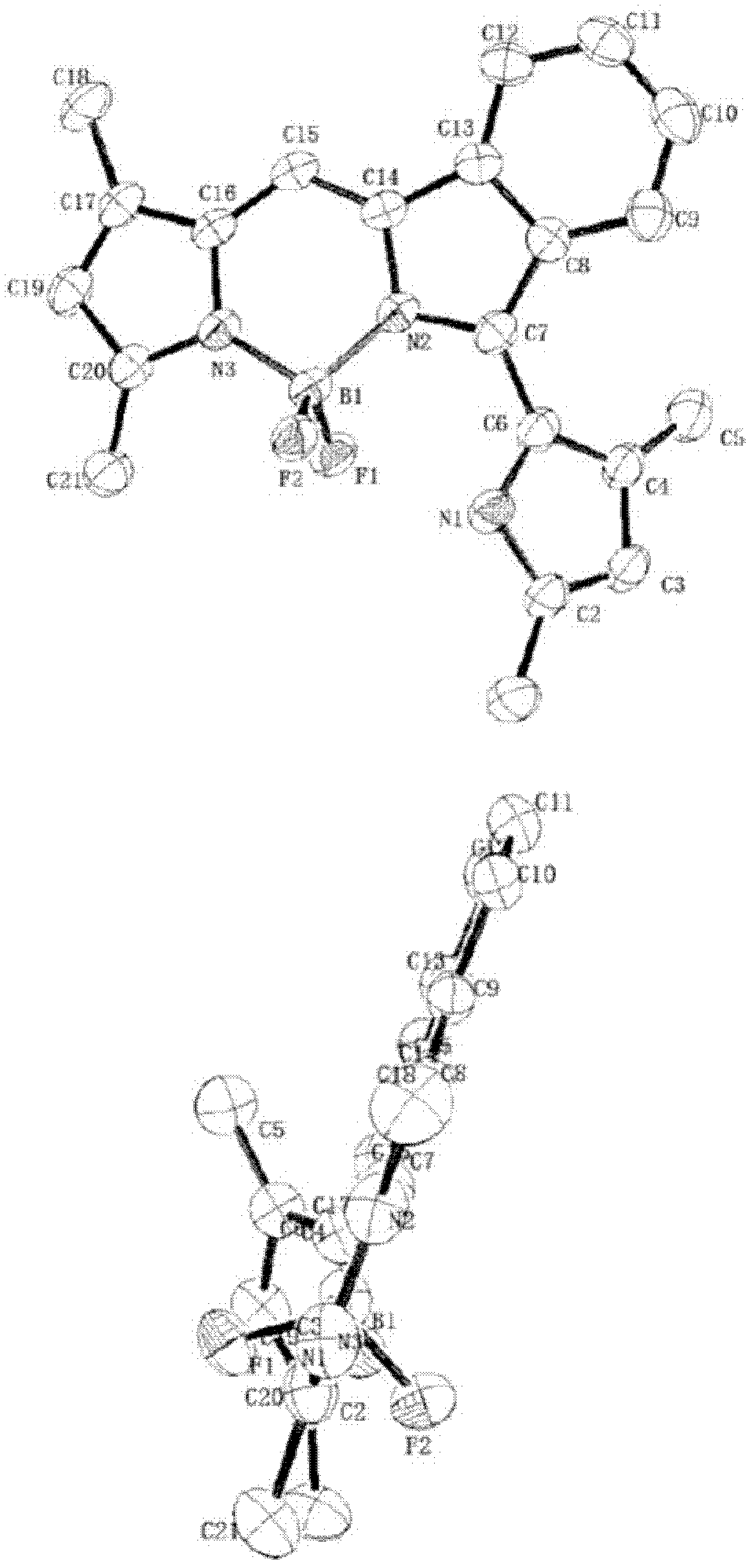
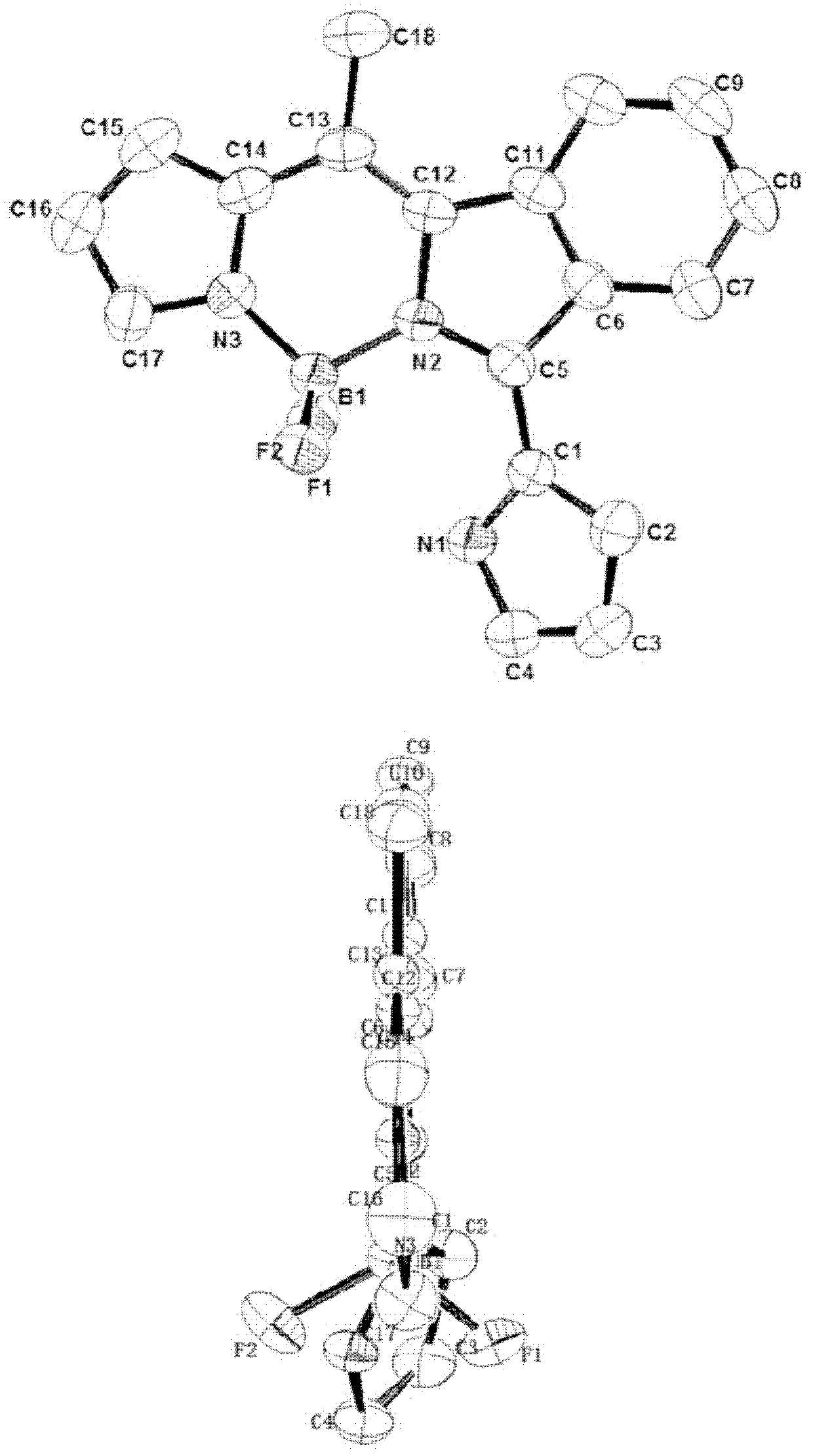
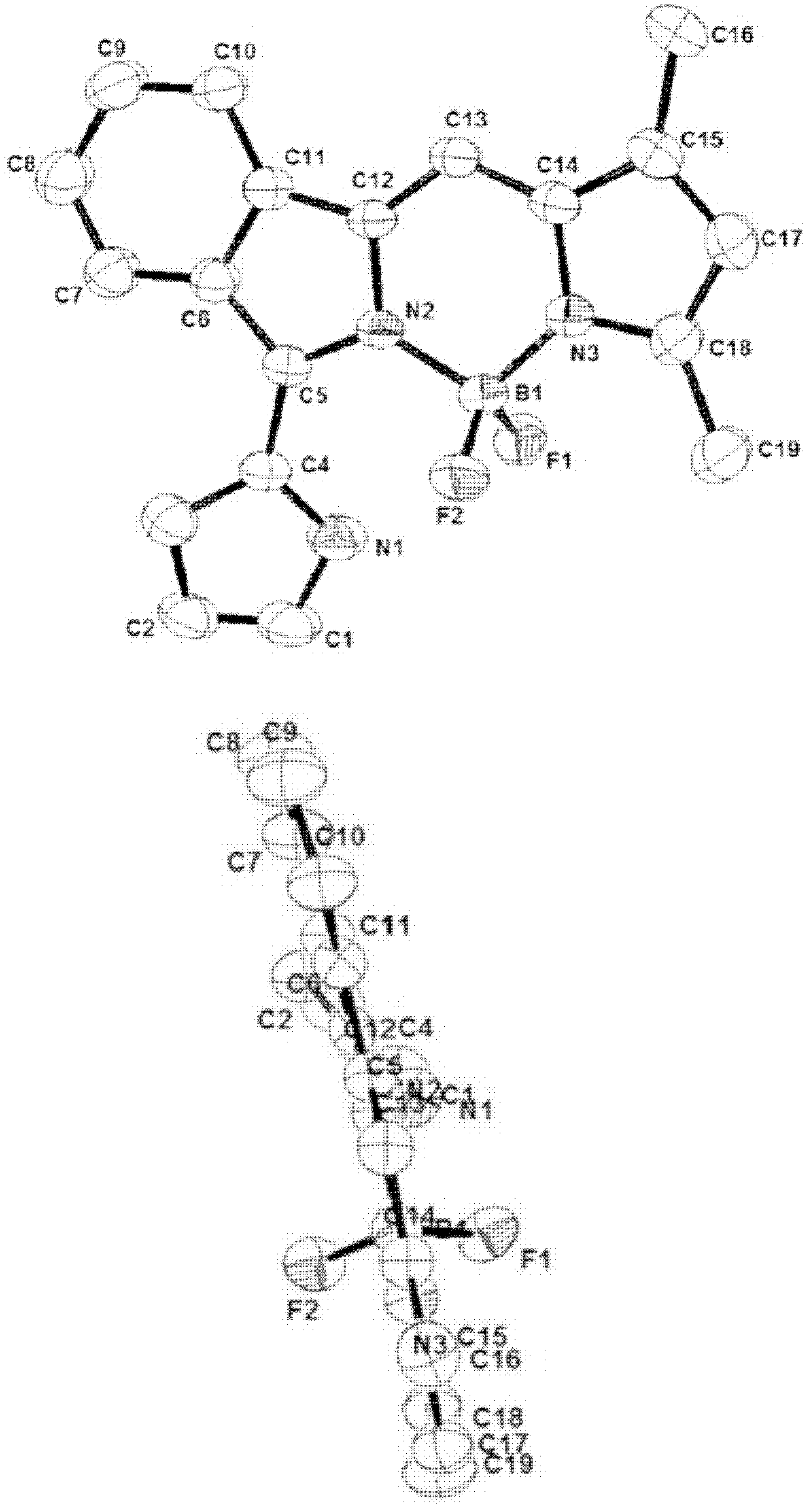
Near infrared fluoro-boron dipyrrole fluorescent dyes and synthesis method thereof
A fluoroboron dipyrrole, fluorescent dye technology, applied in azo dyes, organic dyes, luminescent materials, etc., can solve the problems of harsh reaction conditions, limited types, low luminous efficiency, etc., to increase the conjugation range and optimize photochemistry. The effect of nature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Synthesis of compound IIa:
[0059] method one:
[0060]
[0061] In a 50 ml round bottom flask, add 20 ml of CH under argon 2 Cl 2 , add pyrrole (0.69ml, 10mmol) and 5-chloro-2-aldehyde isoindole (90mg, 0.5mmol) respectively, and add POCl which has been dissolved in 1ml dichloromethane 3 (470μl, 5mmol), after reacting for 16h, transfer to ice bath and add 1.0ml triethylamine, stir at room temperature for 10min, add 1.2ml boron trifluoride diethyl ether in ice water bath, and seal the round bottom flask. Stir overnight at room temperature. After the reaction is over, extract, dry, and concentrate under reduced pressure to obtain the crude product, and then undergo column chromatography (the stationary phase is silica gel, and the eluent is a mixed system of petroleum ether and dichloromethane with a volume ratio of 2 / 1) The dye was isolated as a red solid powder in 35% yield (54 mg).
[0062] Method Two:
[0063]
[0064] In a 50 ml round bottom flask, add ...
Embodiment 2
[0067] Synthesis of Compound IIb:
[0068]
[0069] In a 50 ml round bottom flask, add 20 ml of CH under argon 2 Cl 2 , respectively added 2-methylpyrrole (0.42ml, 5mmol) and 5-chloro-2-aldehyde isoindole (90mg, 0.5mmol), and added POCl which had been dissolved in 1ml dichloromethane 3 (470μl, 5mmol), after reacting for 2h, transfer to ice bath and add 1.0ml triethylamine, after stirring at room temperature for 10min, add 1.2ml boron trifluoride diethyl ether in ice water bath, seal the round bottom flask. Stir at room temperature for 2 hours. After the reaction, extract, dry, and concentrate under reduced pressure to obtain the crude product, and then go through column chromatography (the stationary phase is silica gel, and the eluent is a mixed system of petroleum ether and dichloromethane with a volume ratio of 2 / 1) A dark purple solid powder dye was obtained with a 41% yield (69 mg). 1 H NMR (300MHz, CDCl 3 ): δ10.59(s, 1H), 8.12(d, J=7.8Hz, 1H), 7.78(d, J=7.5Hz, 1H...
Embodiment 3
[0071] Synthesis of compound IIc:
[0072] method one:
[0073]
[0074] In a 50 ml round bottom flask, add 20 ml of CH under argon 2 Cl 2 , respectively added 2,4-dimethylpyrrole (0.5ml, 5mol) and 5-chloro-2-aldehyde isoindole (90mg, 0.5mmol), and added POCl which had been dissolved in 1ml dichloromethane 3 (470μl, 5mmol), after reacting for 2h, transfer to ice bath and add 1.0ml triethylamine, after stirring at room temperature for 10min, add 1.2ml boron trifluoride diethyl ether in ice water bath, seal the round bottom flask. Stir at room temperature for 2 hours. After the reaction, extract, dry, and concentrate under reduced pressure to obtain the crude product, and then go through column chromatography (the stationary phase is silica gel, and the eluent is a mixed system of petroleum ether and dichloromethane with a volume ratio of 2 / 1) A dark purple solid powder dye was obtained in 43% yield (78 mg).
[0075] Method Two:
[0076]
[0077] In a 50 ml round bo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com