Tumor targeting photosensitizers and preparation methods and applications of photosensitizers
A technology of photosensitizer and reaction, which is applied in the field of tumor-targeted photosensitizer and its preparation and application, and can solve the problems of low molar absorbance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
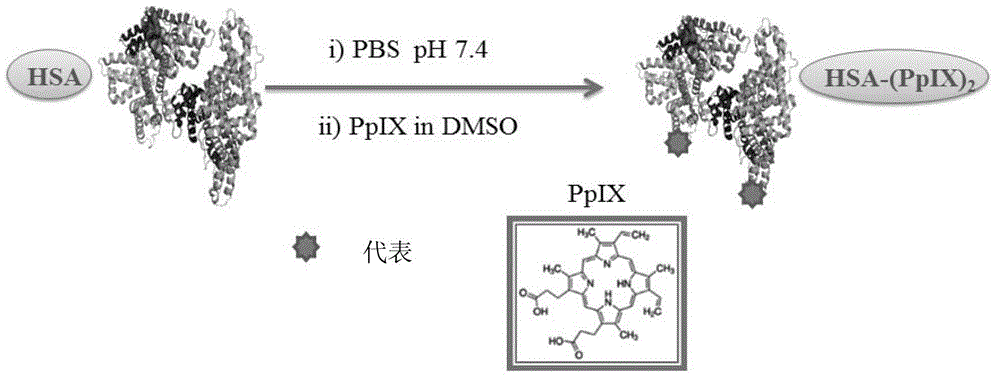
[0036] Example 1: HSA-(PpIX) 2 Preparation process of photosensitizer
[0037] figure 1 HSA-(PpIX) 2 Schematic diagram of the preparation process
[0038] 1) Dissolve 100mg HSA in 5mL PBS (pH7.4) buffer solution, directly add 500μL PpIX DMSO solution with a concentration of 10mM in batches, use 0.1M NaOH solution to adjust the pH to about 7, and react at room temperature for 4h;
[0039] 2) After the reaction, use a dialysis bag with MW=12,000-14,000 to remove small molecules by dialysis, and finally use Sephadex G-25 (PD-10 mesh) for further separation and purification, eluent: 0.5M CH 3 COONa (pH=6.0), using a UV-Vis spectrophotometer at 365nm to monitor the absorption spectrum of PpIX in the dialysate in real time to ensure complete purification and obtain HSA-(PpIX) 2 Photosensitizer.
Embodiment 2
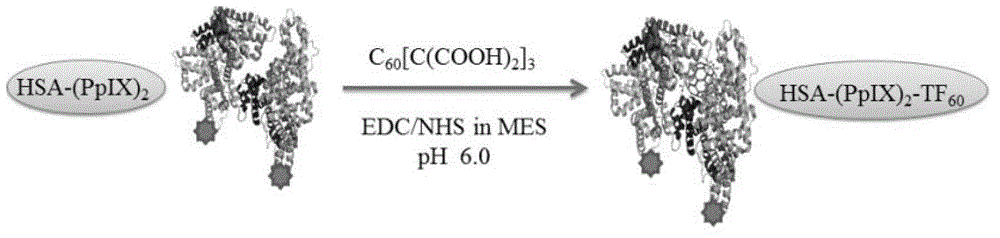
[0040] Example 2: HSA-(PpIX) 2 -TF 60 Preparation process of photosensitizer
[0041] figure 2 HSA-(PpIX) 2 -TF 60 Schematic diagram of the preparation process
[0042] a) (C 60 [C(COOH) 2 ] x (x=3), referred to as TF 60 ) Preparation: reference (Bingel, C., Cyclopropanierung von Fullerenen. Chemische Berichte 1993, 126 (8), 1957-1959.) to prepare fullerene carboxylate derivatives, followed by hydrolysis with NaH to obtain fullerene carboxylate derivatives.
[0043] b) 10 mg fullerene carboxylic acid derivative (C 60 [C(COOH) 2 ] x (x=3)) was dissolved in 10mL MES (pH5.5) buffer, added 10mg EDC and 5mg NHS (C 60 [C(COOH) 2 ] x With EDC, NHS molar ratio is 1:6:6), react at room temperature for 2h to get fullerene carboxylic acid active ester.
[0044] c) 10mg HSA-(PpIX) 2 Add it to the above-mentioned fullerene carboxylic acid active ester solution, stir overnight at room temperature, use a dialysis bag with MW=12,000-14,000 to remove small molecules, and then ...
Embodiment 3
[0048] Example 3: Photodynamic effect evaluation of photosensitizer in vivo
[0049] 1. In vitro dark toxicity test of photosensitizer:
[0050] 5x10 4 Hela cells were seeded into a 96-well plate and cultured overnight. After adding a series of concentration gradient samples and incubating the cells for 24 hours, the cytotoxicity was analyzed using WST-8. 10 μl CCK-8 was added to each well, and an enzyme-linked immunosorbent assay was used at a wavelength of 450nm. The light absorbance value can be determined indirectly, which can indirectly reflect the number of living cells.
[0051] The cell viability of samples incubated with HeLa cells for 24h in the concentration range of 0-50μM was as follows: Figure 6 As shown, cells without sample and PpIX (dissolved in 2 μL DMSO) treated cells were used as controls. The use of HSA-modified photosensitizers significantly increased the biocompatibility of PpIX without apparent cytotoxicity at a concentration of 50 μM. The addition...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com