(METH)acrylic oligomers
An acrylic, oligomer technology, used in ester copolymer adhesives, carboxylate preparation, preparation of organic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] A 1500 mL glass reactor equipped with a heating jacket, stirrer, reflux condenser, feed vessel and nitrogen inlet was charged with 800 g of tert-butyl methacrylate. Next, 160 mg of the cobaloxime catalyst prepared as described above was dissolved in 20 g of ethyl acetate, filtered, and the liquid was added to the reactor. Next, 130 grams of ethyl acetate and 50 grams of acetone were also charged to the reactor, stirring was started, and the total mixture was heated to reflux conditions of about 85°C. An initiator solution consisting of 0.8 grams of 2,2'-azobis(isobutyronitrile) (AIBN) dissolved in 30 grams of ethyl acetate was prepared and added to the reactor over approximately 20 minutes. The reaction mixture was maintained at about 80-87°C for about 2 hours and another initiator solution consisting of 0.8 grams of AIBN and 30.0 grams of ethyl acetate was added over about 20 minutes.
[0079] The reaction was maintained at about 80-85°C for an additional 2 hours and ...
Embodiment 2 to 28
[0081] Several additional disclosed oligomers were prepared in a manner similar to that described in Example 1 above. The only difference between the procedure for preparing the disclosed oligomers in Examples 2-28 and the procedure for preparing the disclosed oligomers in Example 1 is the concentration of cobaloxime (ppm by weight of monomer) and / or Starting monomer composition. Example 11 was synthesized from a different batch of catalyst with increased activity leading to the desired M z Less catalyst is required.
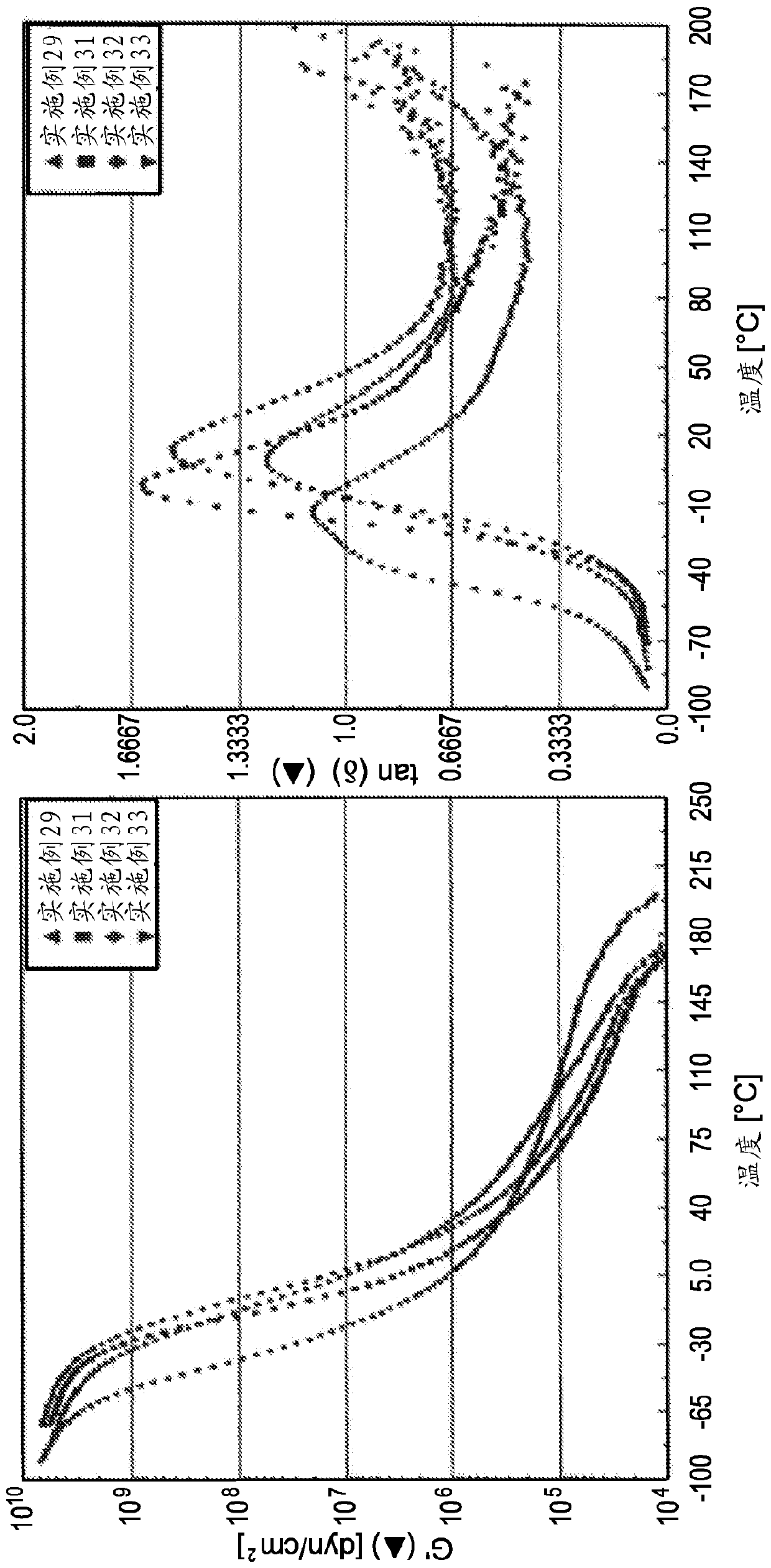
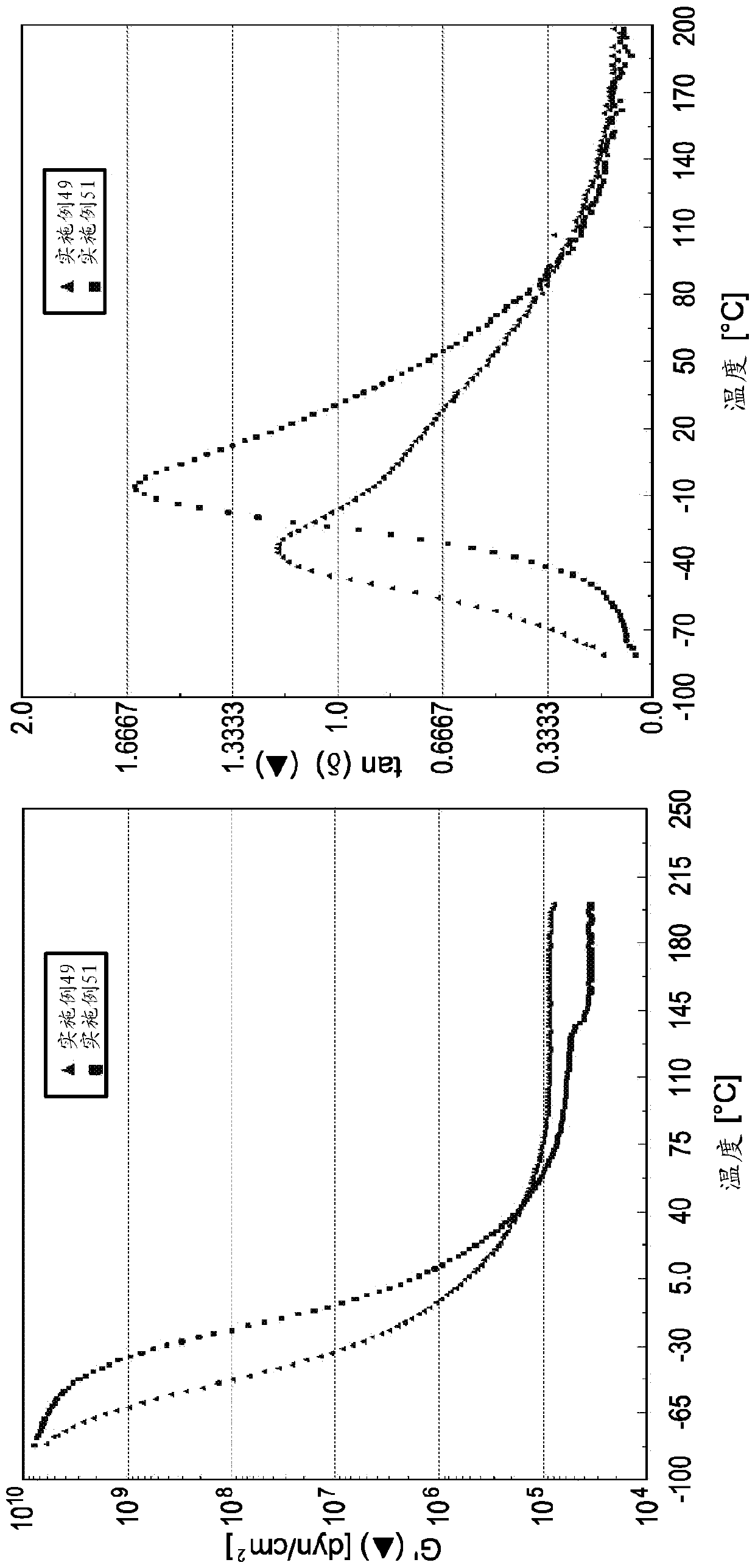
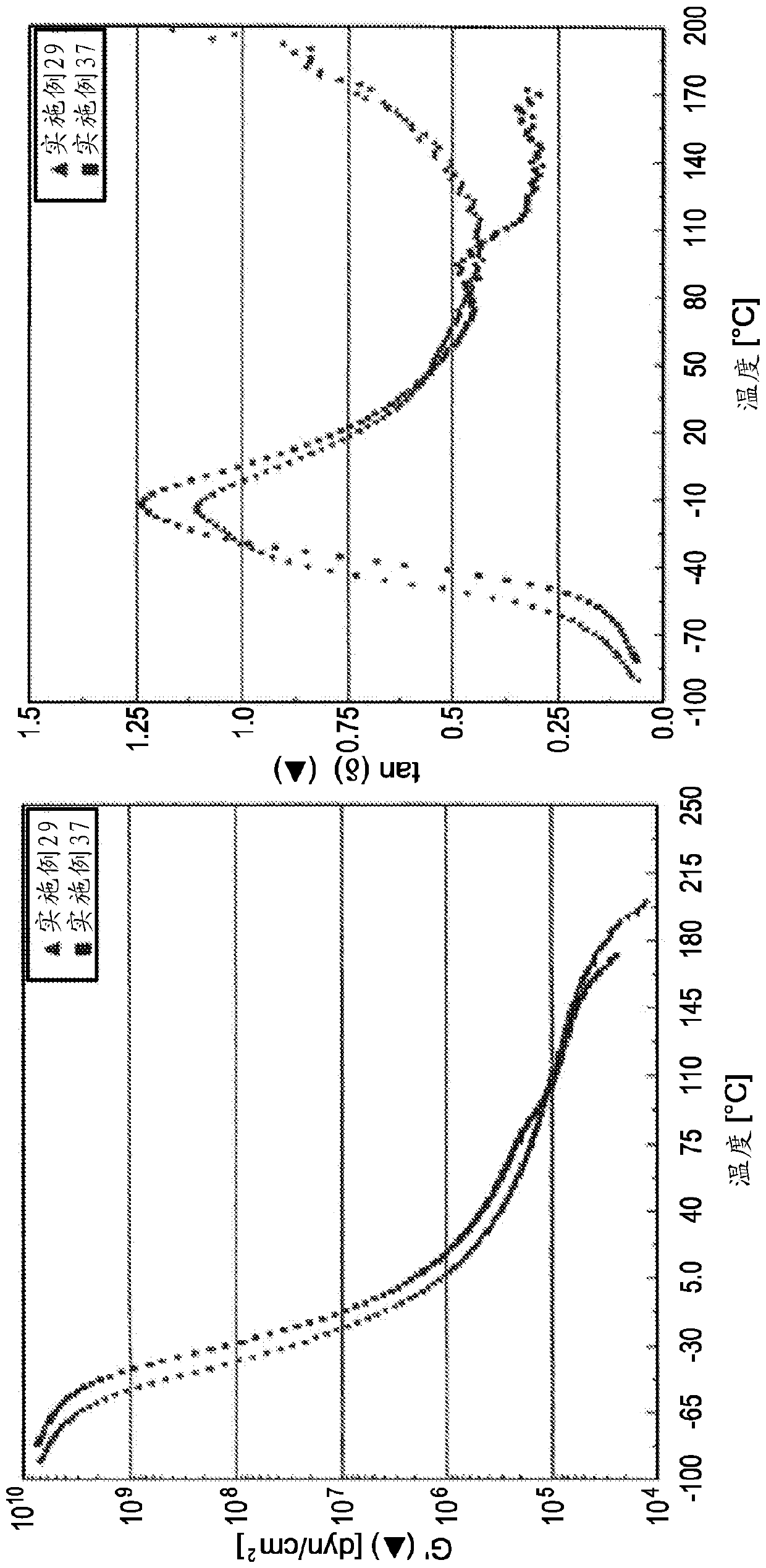
[0082] The molecular weight and polydispersity index of each oligomer prepared in Examples 2-28 were measured and the results are summarized in Table 2 below. In addition, the ring and ball softening points of several oligomers prepared in Examples 2-28 were measured and these values are also provided in Table 2. The oligomer samples prepared in Examples 2-10 were combined with the pressure sensitive adhesive (PSA 1 ) described in Examples 29-39 below.
[...
Embodiment 29 to 39
[0086] by charging 55.0 g of ethyl acetate, 82.3 g of 2-ethylhexyl acrylate, and 4.33 g of acrylic acid into a 1000-mL glass reactor equipped with a heating jacket, stirrer, reflux condenser, feed vessel, and nitrogen inlet. Prepare a model solution acrylic pressure-sensitive adhesive composition (PSA 1). The resulting mixture was then stirred and heated to a reflux temperature of approximately 80°C. An initiator solution was prepared by dissolving 0.17 grams of lauroyl peroxide in 5.0 grams of toluene and charged to the reactor. In a separate feed vessel, 247.0 grams of 2-ethylhexyl acrylate, 13.0 grams of acrylic acid, 260 grams of ethyl acetate, and 0.17 grams of lauroyl peroxide were mixed and fed into the reactor over 2 hours while the temperature was maintained at A reflux temperature of about 80°C. After the feeds, the reaction was maintained at a reflux temperature of approximately 80°C. In a separate feed vessel, 0.7 grams of lauroyl peroxide was dissolved in 70.0 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com