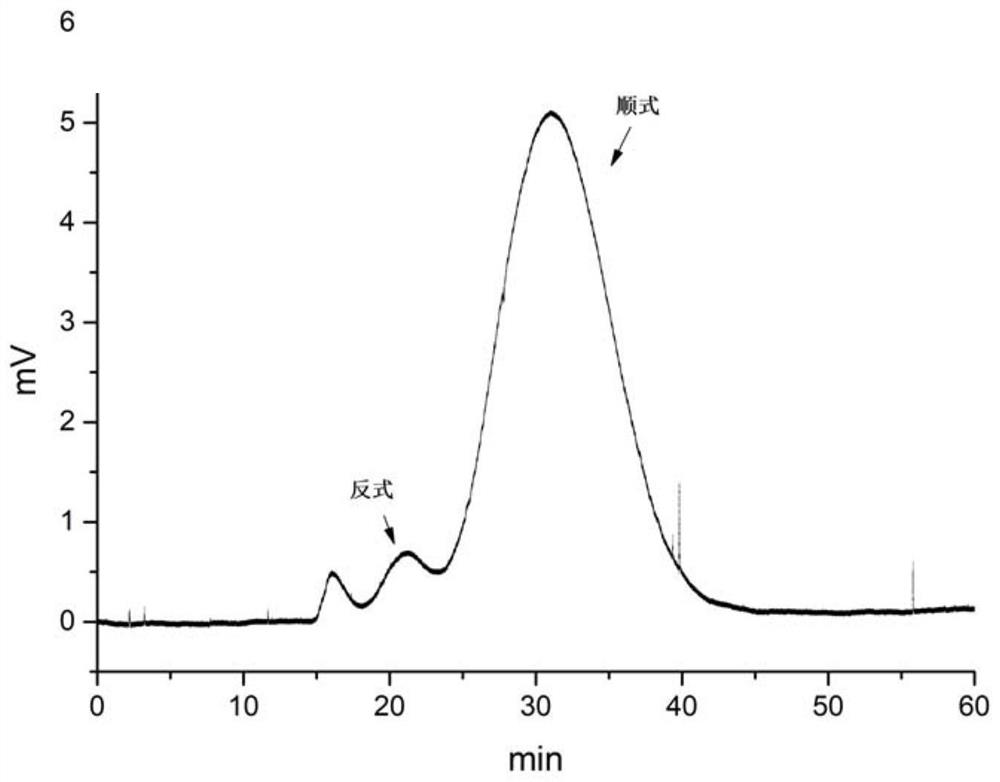
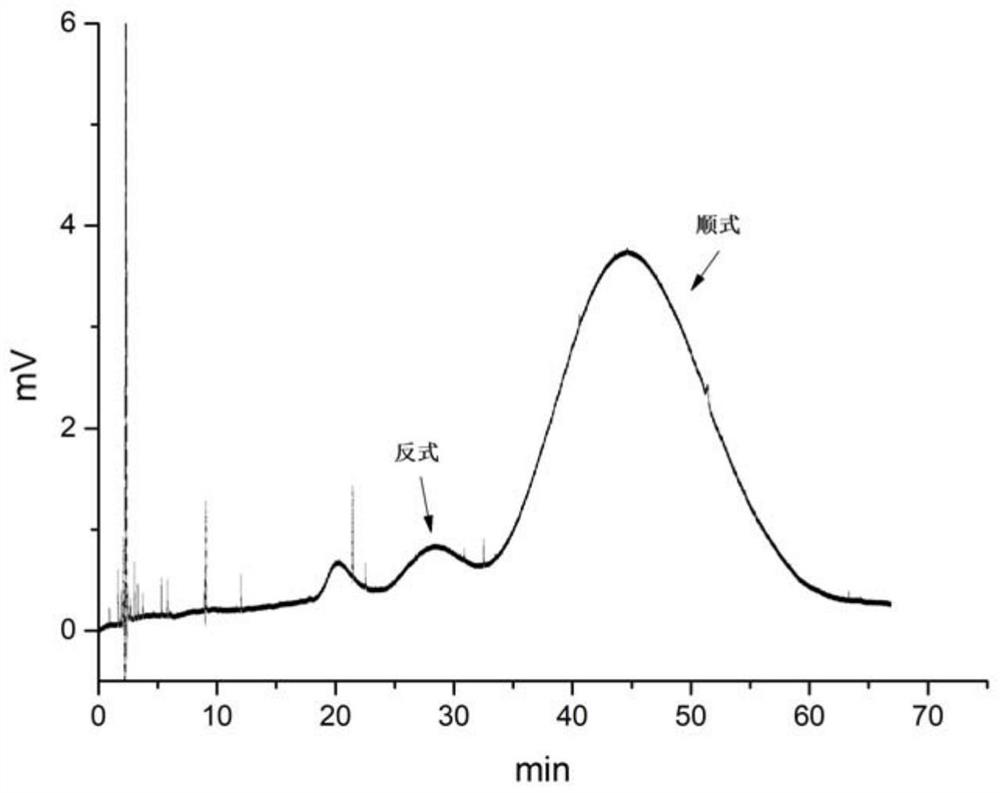
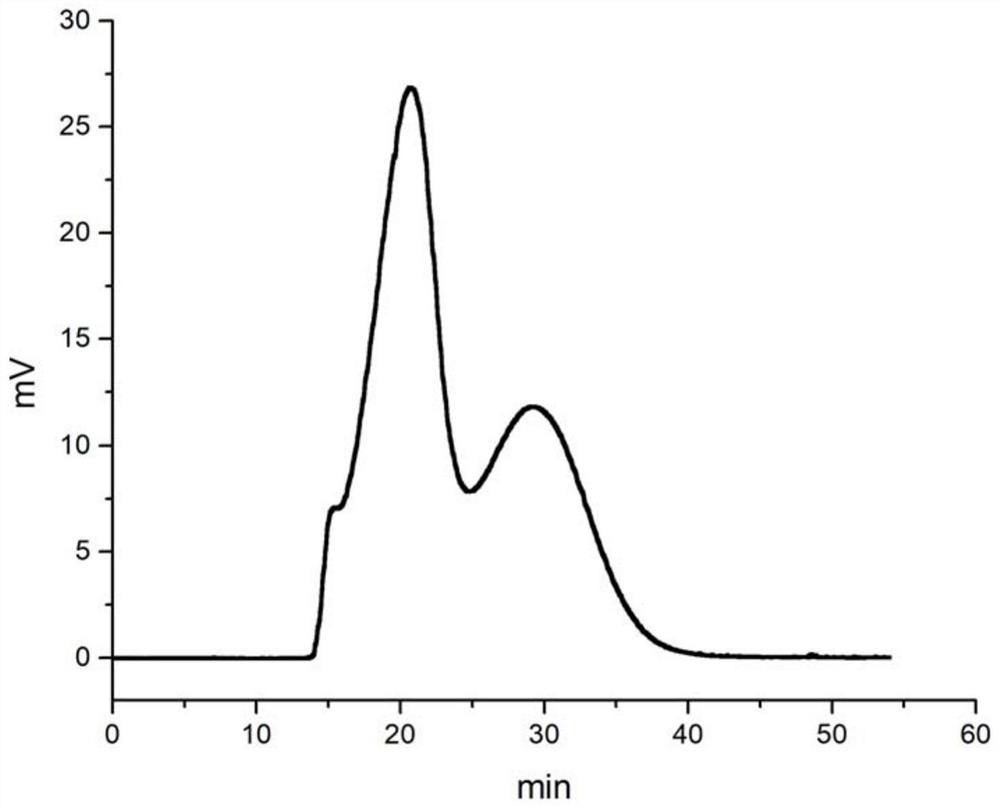
A kind of method of high-speed countercurrent chromatographic separation of cis-trans isomers of sertraline hydrochloride
A technology of high-speed countercurrent chromatography and sertraline hydrochloride, which is applied in the field of high-speed countercurrent chromatography separation, can solve the problem of high cost, and achieve the effects of mild conditions, high economic benefits, and large separation and preparation volume.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Mix 93.65ml 0.10mol / L citric acid aqueous solution with 6.35ml 0.20mol / L disodium hydrogen phosphate aqueous solution to obtain a citric acid-disodium hydrogen phosphate buffer solution with pH=7.6, then add 15.07g hydroxypropyl-β - Cyclodextrin is mixed to obtain a citric acid-disodium hydrogen phosphate buffer solution containing hydroxypropyl-β-cyclodextrin, wherein the concentration of hydroxypropyl-β-cyclodextrin is 0.10mol / L;
[0036] Then mix n-hexane with citric acid-disodium hydrogen phosphate buffer solution containing hydroxypropyl-β-cyclodextrin according to the volume ratio of 1:1 and place them in a separatory funnel, shake well, let stand to separate layers, and wait for equilibrium After separation, the obtained organic phase was used as the stationary phase, and the aqueous phase was used as the mobile phase.
[0037] (2) Weigh 2 mg of racemic sertraline hydrochloride (mainly cis-sertraline hydrochloride, synthetic method references: Peng Dongming, ...
Embodiment 2
[0043](1) Mix 95.75ml 0.10mol / L citric acid aqueous solution with 4.25ml 0.20mol / L disodium hydrogen phosphate aqueous solution to obtain pH=7.8 citric acid-disodium hydrogen phosphate buffer solution, then add 15.07g hydroxypropyl-β- The cyclodextrins are mixed to obtain a citric acid-disodium hydrogen phosphate buffer solution containing hydroxypropyl-β-cyclodextrin, wherein the concentration of hydroxypropyl-β-cyclodextrin is 0.10mol / L;
[0044] Then mix n-hexane with citric acid-disodium hydrogen phosphate buffer solution containing hydroxypropyl-β-cyclodextrin according to the volume ratio of 1:1 and place them in a separatory funnel, shake well, let stand to separate layers, and wait for equilibrium After separation, the obtained organic phase was used as the stationary phase, and the aqueous phase was used as the mobile phase.
[0045] (2) Weigh 2 mg of racemic sertraline hydrochloride and dissolve it in 1 ml of water phase, fully dissolve to make a sample solution for ...
Embodiment 3
[0051] (1) Mix 93.65ml 0.10mol / L citric acid aqueous solution with 6.35ml 0.20mol / L disodium hydrogen phosphate aqueous solution to obtain a citric acid-disodium hydrogen phosphate buffer solution with pH=7.6, then add 15.07g hydroxypropyl-β- The cyclodextrins are mixed to obtain a citric acid-disodium hydrogen phosphate buffer solution containing hydroxypropyl-β-cyclodextrin, wherein the concentration of hydroxypropyl-β-cyclodextrin is 0.10mol / L;
[0052] Then mix n-hexane with citric acid-disodium hydrogen phosphate buffer solution containing hydroxypropyl-β-cyclodextrin according to the volume ratio of 1:1 and place them in a separatory funnel, shake well, let stand to separate layers, and wait for equilibrium After separation, the obtained organic phase was used as the stationary phase, and the aqueous phase was used as the mobile phase.
[0053] (2) Weigh 4 mg of racemic sertraline hydrochloride and dissolve it in 1 ml of water phase, fully dissolve to make a sample solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com