Construction method of semen oroxyli formula granule UPLC specific chromatogram and application thereof
A technology of formula granules and characteristic maps, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems that there is no effective method for controlling the quality of wood butterfly formula granules, and achieve the effect of an effective and rapid evaluation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
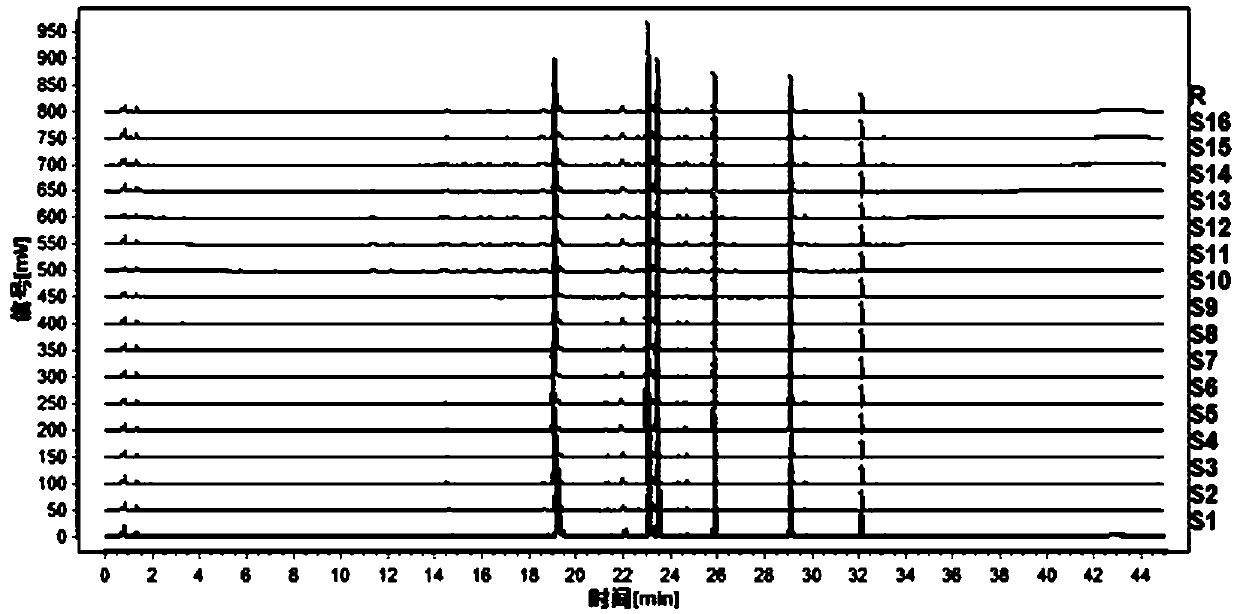
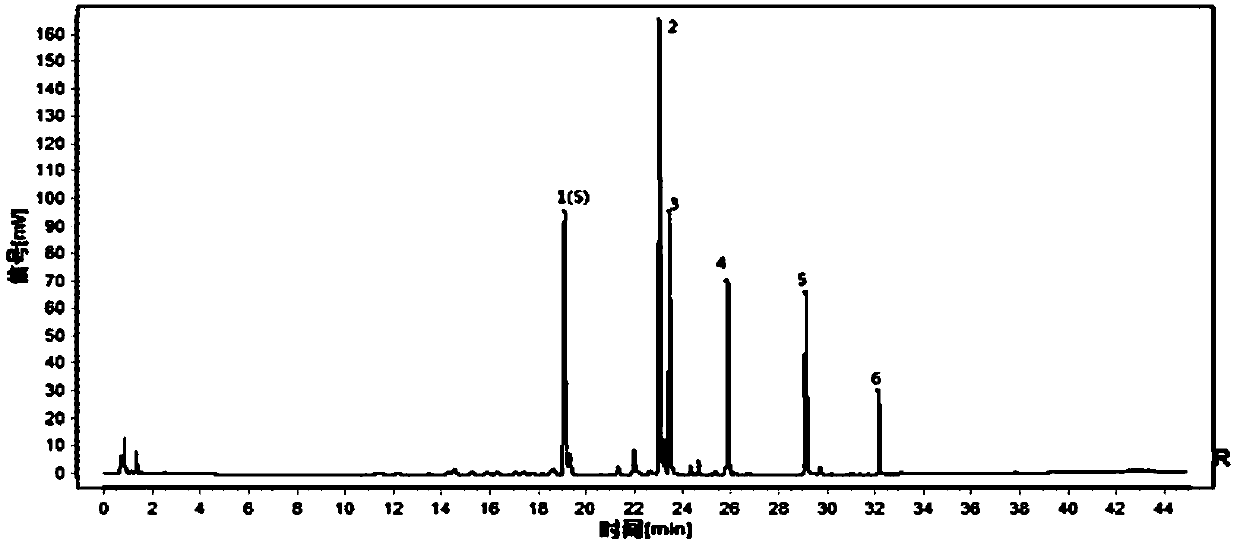
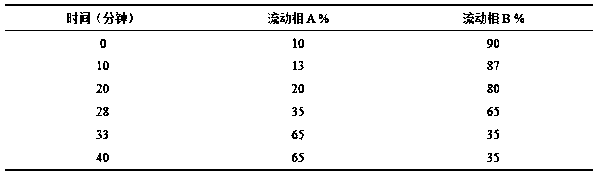
[0031] The present invention will be further described below through specific embodiments. The following examples are specific embodiments of the present invention, but the embodiments of the present invention are not limited by the following examples.
[0032] 1. Instruments and reagents
[0033] 1.1 Instrument
[0034] Waters liquid mass spectrometer (liquid part ACQUITY UPLC H-Class, mass spectrometry part Waters Xevo TQDMS); millionth balance (METTLER TOLEDO, XP26), one millionth balance (METTLER TOLEDO, ME204E), Agilent SB C 18 Chromatographic column (2.1×100mm, 1.8μm), electric heating constant temperature water bath (Shanghai Yiheng Technology Co., Ltd., HWS-28), numerical control ultrasonic cleaner (Kunshan Ultrasonic Instrument Co., Ltd., KQ500DE), ultrapure water system (Merck Incorporated, Milli-Q-Direct).
[0035] 1.2 Reference substances and reagents
[0036] Acetonitrile (Merck, Germany, chromatographically pure); ethanol (Xilong Science Co., Ltd., analytical...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com