Preparation method of porous cobalt carbide
A cobalt carbide and cobalt source technology, applied in chemical instruments and methods, carbon compounds, inorganic chemistry, etc., can solve the problem of difficulty in controlling the size of cobalt tetroxide and sintering agglomeration, uncontrollable particle size and morphology of cobalt carbide products, and large cobalt carbide particle size To achieve the effect of improving the carbonization reaction efficiency, solving the uncontrollable particle size and shape, and avoiding agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
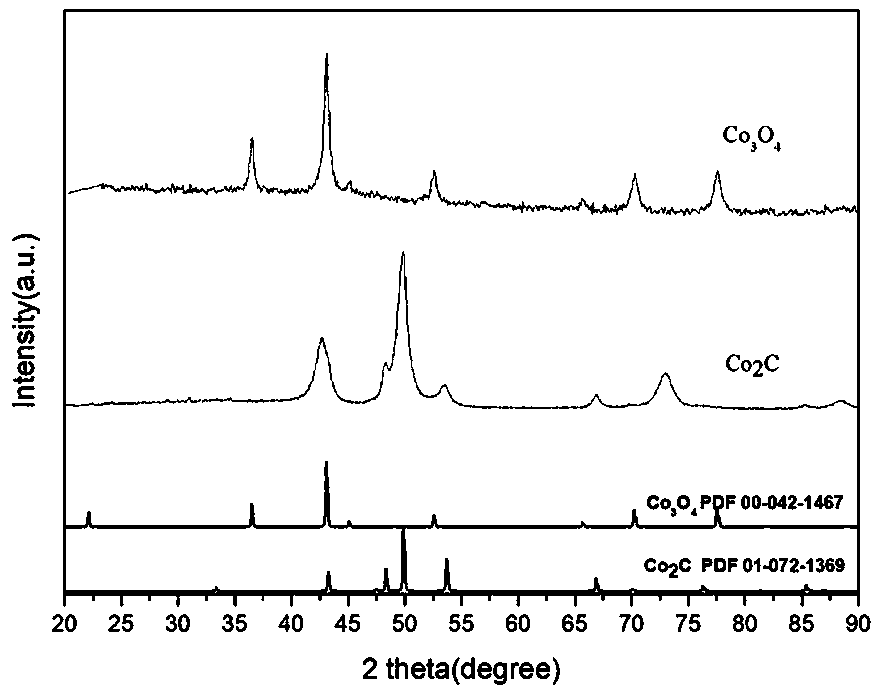
Image
Examples
Embodiment 1
[0034] Weigh 100g of coconut shell activated carbon, pour 200mL of nitric acid solution with a mass fraction of 30%, mechanically stir for 3 hours and then let it stand for 21 hours. After standing, wash the activated carbon repeatedly with deionized water until the pH value of the washing solution is detected to be 7. . The activated carbon after cleaning was put into an oven and dried at 120° C. for 12 hours to obtain pretreated activated carbon, which was set aside. Dissolve 3.58g of cobalt nitrate in 25mL of deionized water to form the first solution, and dissolve 1.42g of ammonium carbonate in 5mL of deionized water to form the second solution. The temperature of the first solution and the second solution is maintained at 35°C. Select 10g of activated carbon that has been treated, and impregnate the first solution and the second solution on the activated carbon for several times: take 7mL of the first solution and impregnate it into the activated carbon, let it stand for ...
Embodiment 2
[0036] Weigh 100g of coconut shell activated carbon, pour 200mL of nitric acid solution with a mass fraction of 30%, mechanically stir for 3 hours and then let it stand for 21 hours. After standing, wash the activated carbon repeatedly with deionized water until the pH value of the washing solution is detected to be 7. . The activated carbon after cleaning was put into an oven and dried at 120° C. for 12 hours to obtain pretreated activated carbon, which was set aside. Dissolve 3.58g of cobalt nitrate in 13mL of deionized water to form the first solution, and dissolve 1.42g of ammonium carbonate in 11.5mL of deionized water to form the second solution. The temperature of the first solution and the second solution is kept at 35°C. Select 10g of activated carbon that has been treated, and impregnate the first solution and the second solution on the activated carbon for several times: take 7mL of the first solution and impregnate it into the activated carbon, let it stand for 30 ...
Embodiment 3
[0038] Weigh 100g of coconut shell activated carbon, pour 200mL of nitric acid solution with a mass fraction of 30%, mechanically stir for 3 hours and then let it stand for 21 hours. After standing, wash the activated carbon repeatedly with deionized water until the pH value of the washing solution is detected to be 7. . The activated carbon after cleaning was put into an oven and dried at 120° C. for 12 hours to obtain pretreated activated carbon, which was set aside. Dissolve 7.16g of cobalt nitrate in 25mL of deionized water to form the first solution, and dissolve 2.84g of ammonium carbonate in 5mL of deionized water to form the second solution. The temperature of the first solution and the second solution is kept at 35°C. Select 10g of activated carbon that has been treated, and impregnate the first solution and the second solution on the activated carbon for several times: take 7mL of the first solution and impregnate it into the activated carbon, let it stand for 30 min...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com