Synthesis method of aggregation pheromone (E)-cis-6, 7-epoxy-2-nonenal of Aromia bungii
A technology for gathering pheromones and pink-necked beetles, which is applied in the new synthesis field of natural product pheromone components, can solve the problems of harsh conditions, expensive raw materials, unsuitable for industrial production, etc., and achieve the effect of simple operation and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
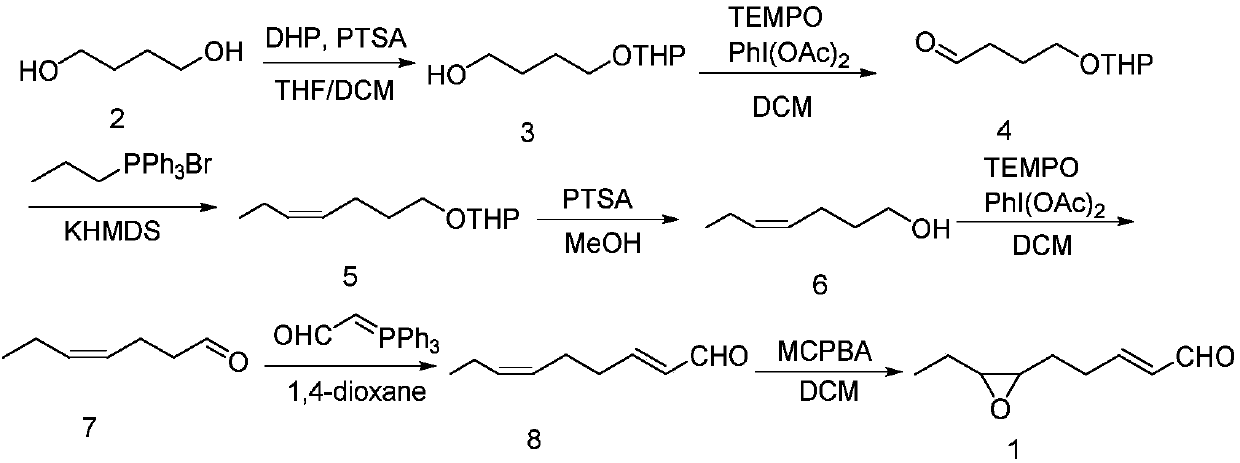
[0027] Example 1: Add p-toluenesulfonic acid monohydrate (2.3g, 12mmol) to a tetrahydrofuran / dichloromethane mixed solution (220mL) of 1,4-butanediol (13.0g, 144mmol), cool to 0°C, drop Add 3,4-dihydro-2H-pyran (10.8mL, 120mmol), and stir at room temperature for 6h. TLC detection, after the reaction was completed, quenched by adding water, extracted with dichloromethane, combined organic phases, dried over anhydrous sodium sulfate, concentrated under reduced pressure, purified by column chromatography to obtain a colorless liquid 3 (15.56g, yield 75% ). 1 H NMR (400MHz, CDCl 3 ): δ4.53(t, J=3.5Hz, 1H), 3.76-3.81(m, 1H), 3.69-3.73(m, 1H), 3.57(t, J=6.0Hz, 2H), 3.43-3.46( m,1H),3.34-3.37(m,1H),2.98(s,1H),1.72-1.76(m,1H),1.61-1.65(m,5H),1.44-1.53(m,4H); 13 C NMR (100MHz, CDCl 3 ): δ99.8, 67.5, 62.4, 62.3, 30.6, 29.8, 26.4, 25.3, 19.5.
Embodiment 2
[0028] Example 2: Pyridinium p-toluenesulfonate (0.31g, 1.2mmol) was added to a mixed solution of 1,4-butanediol (1.3g, 14.4mmol) in tetrahydrofuran dichloromethane (22mL), and after cooling to 0°C, Add 3,4-dihydro-2H-pyran (1.1 mL, 12 mmol) dropwise and stir at room temperature for 6 h. TLC detection, after the reaction was completed, quenched by adding water, extracted with dichloromethane, combined organic phases, dried over anhydrous sodium sulfate, concentrated under reduced pressure, purified by column chromatography to obtain a colorless liquid 3 (1.5g, yield 70% ). Compared with Example 1, p-toluenesulfonic acid monohydrate was changed to p-toluenesulfonic acid pyridinium salt, and other conditions were unchanged. product of 1 H NMR and 13 CNMR is completely consistent with Example 1.
Embodiment 3
[0029]Example 3: Add p-toluenesulfonic acid monohydrate (0.23g, 12mmol) to a mixed solution of 1,4-butanediol (1.08g, 12mmol) in tetrahydrofuran dichloromethane (22mL), after cooling to 0°C, drop Add 3,4-dihydro-2H-pyran (1.1 mL, 12 mmol), and stir at room temperature for 6 h. TLC detection, after the reaction was completed, quenched by adding water, extracted with dichloromethane, combined organic phases, dried over anhydrous sodium sulfate, concentrated under reduced pressure, purified by column chromatography to obtain a colorless liquid 3 (1.3g, yield 62% ). Compared with Example 1, the equivalent of 1,4-butanediol is reduced, and other conditions remain unchanged. product of 1 H NMR and 13 C NMR is completely consistent with Example 1.
[0030] Step 2: Preparation of 4-((tetrahydro-2H-pyran-2-yl)oxy)butyraldehyde (4)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com