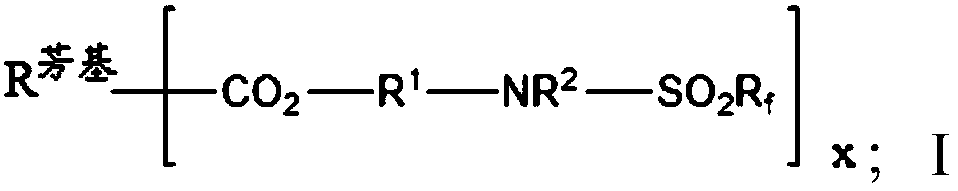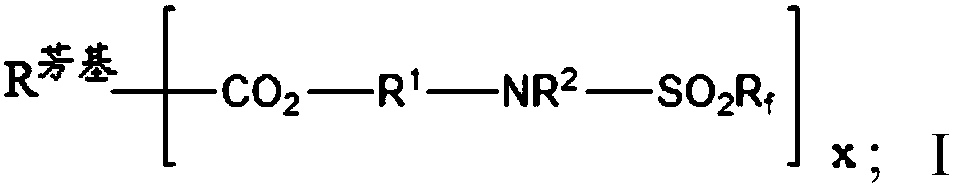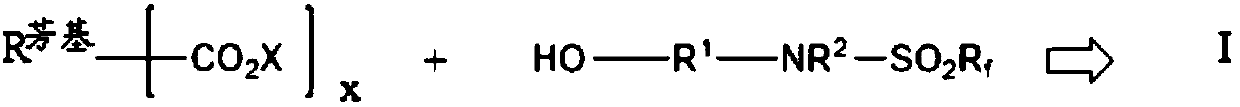Partially fluorinated aromatic esters
A technology of aryl and composition, applied in the field of polymer composition, can solve the problems of expensive, poor thermal stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0089] Material
[0090] All chemicals were obtained or are generally commercially available from chemical companies such as Sigma-Aldridge Chemical Company, St. Louis, MO, unless otherwise indicated.
[0091] C18 diester (C 4 f 9 SO 2 N(CH 3 )CH 2 CH 2 OOC (CH 2 ) 16 COOCH 2 CH 2 (CH 3 ) NSO 2 C 4 f 9 ) was prepared by the method described in F-4 of the following US Patent No. 7,396,866 B2.
[0092] method
[0093] Water repellency test method
[0094] For the water repellency test, film samples prepared according to the Examples and Comparative Examples described below were placed on a flat, horizontal surface. Five small drops of water or a water / isopropanol (IPA) mixture were gently placed on a spot at least two inches (approximately 5 cm) away from the film sample. The film sample was considered to pass the test if four out of five drops were visible as spheres or hemispheres after ten seconds of viewing at a 45° angle. The reported water repellenc...
preparation example 1
[0098] Preparation Example 1 (PE1)
[0099]
[0100] 1,1,2,2,3,3,4,4,4-nonafluoro-N-(2-hydroxyethyl)-N-methyl-butane-1-sulfonamide (249g, 697.11mmol) , methanesulfonic acid (1.73mL, 26.1mmol), isobenzofuran-1,3-dione (51.5g, 348mmol) and toluene (174mL, 1630mmol) were added to a 500mL circle equipped with a magnetic stirring bar and reflux condenser in the bottom flask. The reaction mixture was stirred at 110 °C for 18 hours. Triethylamine (3.67 mL, 26.1 mmol) was then added to the resulting pale yellow solution, and the reaction mixture was allowed to cool to room temperature. The resulting precipitate was collected by filtration, washed with toluene, and dried under vacuum to afford benzene-1,2-dicarboxylic acid bis[2[methyl(1,1,2,2,3,3, 4,4,4-Nafluorobutylsulfonyl)amino]ethyl]ester (273 g, 323.3 mmol, 93.0% yield). The identity of the material was confirmed by LC / MS and NMR techniques.
preparation example 2
[0101] Preparation example 2 (PE2)
[0102]
[0103]1,1,2,2,3,3,4,4,4-nonafluoro-N-(2-hydroxyethyl)-N-methylbutane-1-sulfonamide (2.01 equivalents, 566mmol), Triethylamine (2.2 equivalents, 620 mmol) and toluene (0.5 M, 5280 mmol) were added to a mixture equipped with a mechanical stirrer, reflux condenser and benzene-1,3-dicarbonyl chloride dissolved in a mixture of toluene and dichloromethane ( 57.2g, 282mmol) in a 1L 3-neck flask with an addition funnel. Dissolved ethanol was added to the stirred reaction mixture under positive nitrogen pressure. An exotherm was observed and a white precipitate started to form. After the addition was complete, the reaction mixture was allowed to stir at room temperature for 18 hours, at which time water (300 mL) was added. The mixture was allowed to stir for 5 minutes, and the resulting white solid was collected by filtration and washed sequentially with dichloromethane (300 mL, 3x) and water (300 mL, 3x). The resulting white powde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com