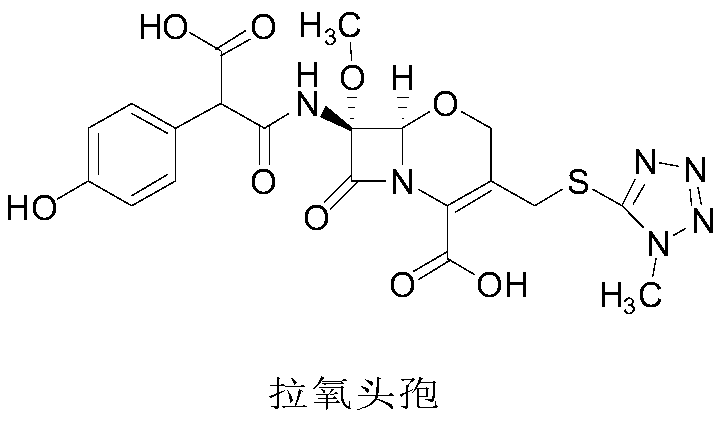
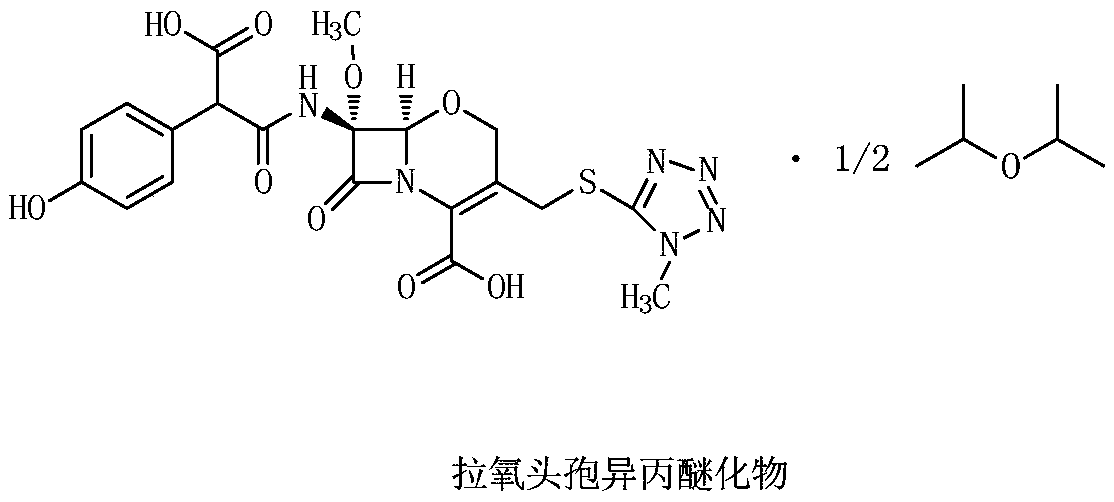
Latamoxef isopropyl etherate as well as preparation method and application of latamoxef isopropyl etherate
A technology of latamoxef isopropyl ether and latamoxef, applied in the field of drug synthesis, can solve the problems of difficult drying, unstable product quality, difficult filtration, etc., and achieve the effects of not easy to absorb moisture, not easy to deteriorate, and easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Drop 2.0L of 0.20g / mL Latamoxefamic acid methyl ester solution (0.5% water content) into 30L isopropyl ether at room temperature, stir and crystallize, filter, and store the wet product at 40°C with a vacuum of -0.09 Baking under MPa for 10 h gave 428.57 g of Latamoxyceph isopropyl ether compound with a yield of 97.5%, a Latamoxycef purity of 98.7%, a maximum impurity of 0.20%, and a total impurity of 1.3%.
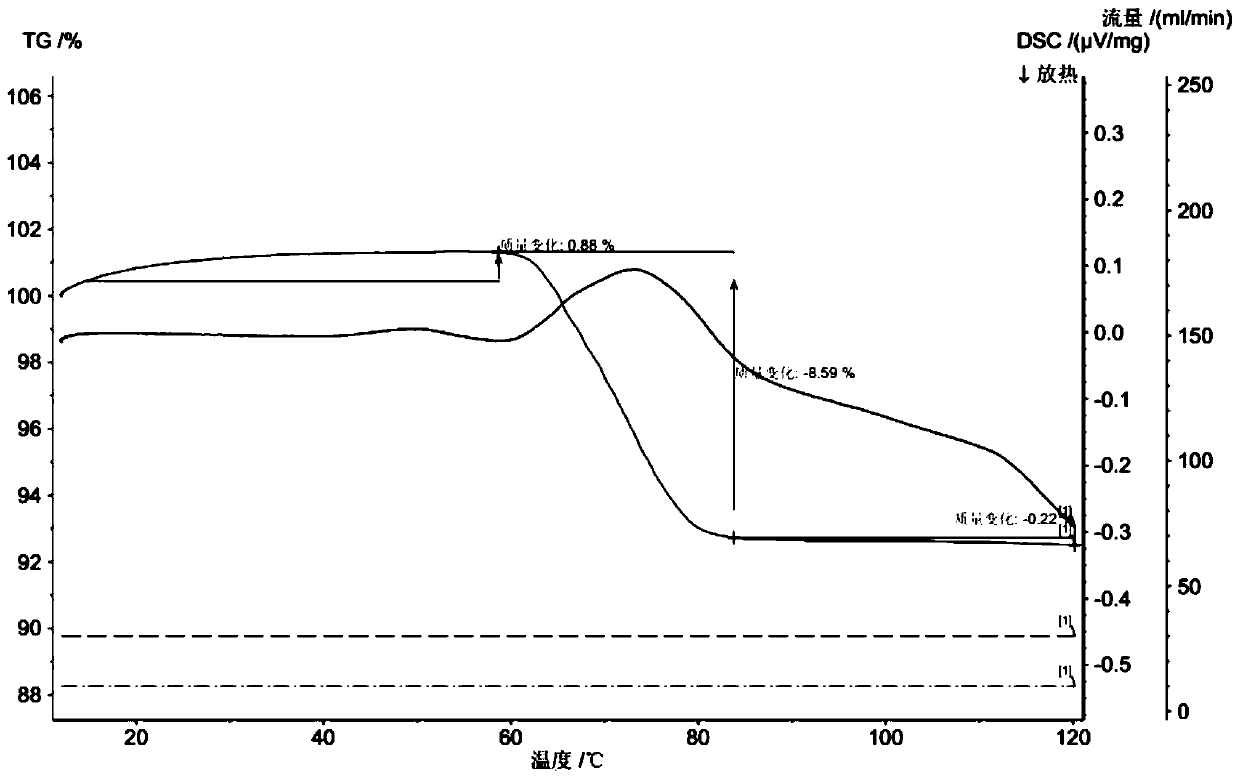
[0044] The thermogravimetric analysis spectrogram (TG spectrogram) and differential scanning calorimetry (DSC spectrogram) that above-mentioned Latamoxef isopropyl ether compound is measured, such as figure 1 shown by figure 1 It can be seen that the Latamoxef isopropyl ether compound has a characteristic endothermic peak at 58°C to 83°C, and loses 8.59% of its weight at 58°C to 83°C, and the number of isopropyl ethers is about 0.5.
Embodiment 2~5
[0046] Change Latamoxef dissolving solvent kind to embodiment 1, use different amounts of isopropyl ether, obtain the result of following table:
[0047]
Embodiment 6
[0049] Drop 2.0L of latamoxycefide acetone solution (water content 0.8%) with a concentration of 0.20g / mL into 90L of isopropyl ether at -30°C, stir and crystallize, filter, and store the wet product at 10°C with a vacuum of -0.10 Baking under MPa for 5h gave 430.77g of Latamoxyceph isopropyl ether compound with a yield of 98.0%, a Latamoxycef purity of 98.1%, a maximum impurity of 0.30%, and a total impurity of 1.9%.
[0050] The DSC spectrogram and TG spectrogram of the Latamoxycefe diprofen compound obtained in this example are consistent with the spectrogram results obtained in Example 1 within the error range. The latamoxef isopropyl ether compound has a characteristic endothermic peak at 58°C to 83°C, and has a weight loss of 8.85% at 58°C to 83°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com