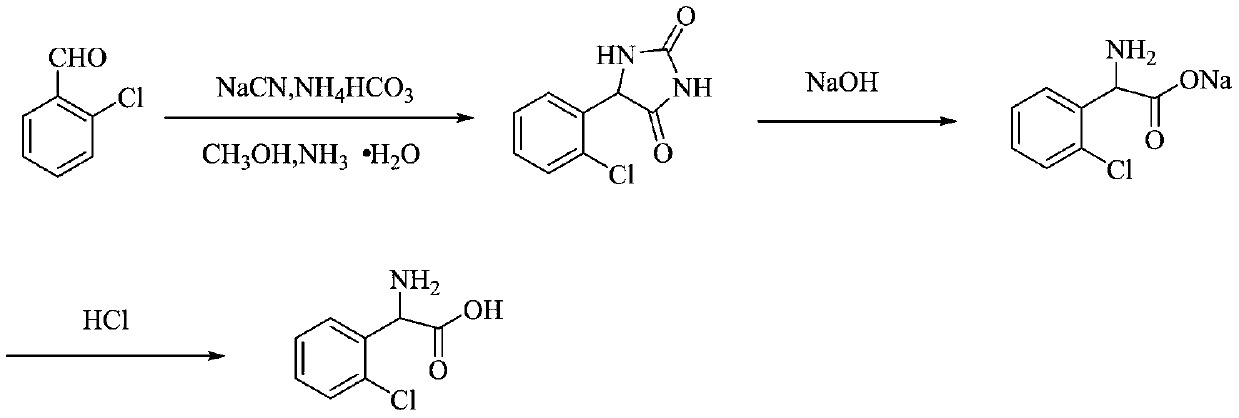
Preparation method of 2-chlorophenylglycine
A technology of o-chlorophenylglycine and o-chlorobenzaldehyde is applied in the preparation of organic compounds, chemical instruments and methods, preparation of cyanide reaction, etc., and can solve the problems of difficulty in recycling ammonia water, high cost of recovering methanol, and high equipment requirements, To achieve the effect of reducing post-processing pressure, reducing production costs, and environmental protection in the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
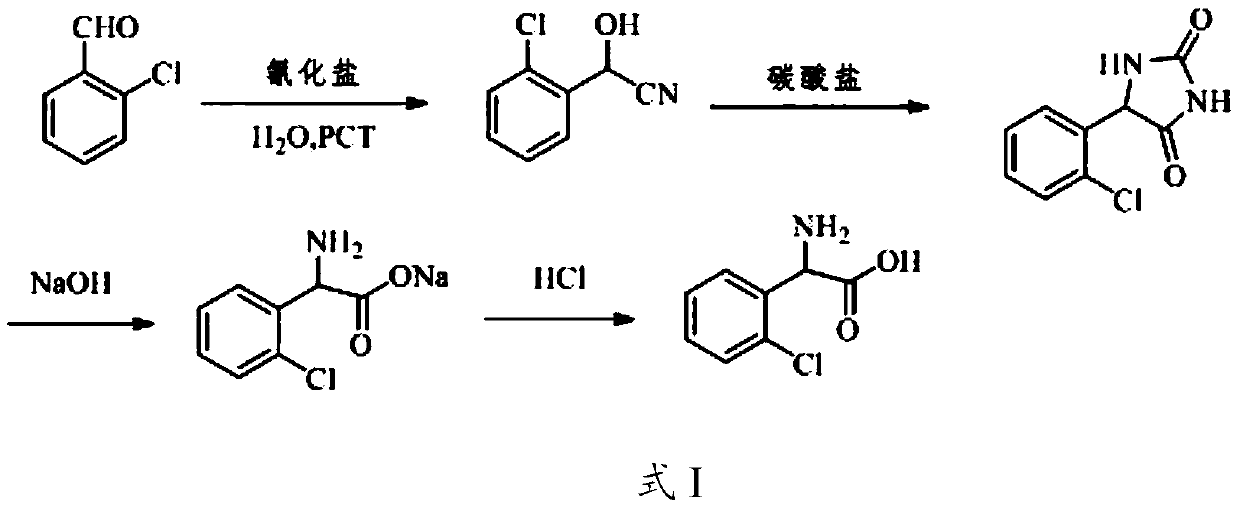
[0029] The invention provides a kind of preparation method of o-chlorophenylglycine, comprising the following steps:
[0030] (1) water, cyanide salt solution and o-chlorobenzaldehyde are mixed, carry out nucleophilic addition reaction under the effect of phase-transfer catalyst, obtain the nucleophilic addition reaction liquid comprising 2-chloro-α-hydroxyphenylacetonitrile;
[0031] (2) the nucleophilic addition reaction solution obtained in step (1) is mixed with carbonate to carry out the Bucherer-Berger reaction, to obtain the Bucherer-Berger reaction solution comprising o-chlorophenylhydantoin;
[0032] (3) The Bucherer-Berg reaction solution obtained in step (2) is subjected to alkali hydrolysis, decolorization and acidification in sequence to obtain o-chlorophenylglycine.
[0033] The concrete reaction process of preparation method provided by the invention is as shown in formula I:
[0034]
[0035] The present invention mixes water, cyanide salt solution and o-ch...
Embodiment 1
[0046] Water (200 mL), tetrabutylammonium bromide (5.7 g) and 30% sodium cyanide solution (70.0 g) were added to a 500 mL three-necked flask, and the system was stirred at room temperature for 30 min. O-chlorobenzaldehyde (50.0 g) was added to the system. After the addition was complete, the system was slowly warmed up to 35° C. for 4 hours. After the reaction was completed, ammonium bicarbonate (79.0 g) was added to the system. After the addition was complete, the system continued to react at 55° C. for 4 h.
[0047] After the reaction is finished, add 30% liquid caustic soda to the system to adjust the pH value of the system to ≥12. Then the reaction solution was transferred into a self-boosting high-pressure reactor, the temperature of the system was raised to 135° C., the pressure was 0.3 MPa, and the reaction was carried out for 5 hours.
[0048] After the reaction, the temperature of the system was lowered to 70° C., activated carbon (1 g) was added, and the mixture was...
Embodiment 2
[0050] Water (200 mL), trimethylbenzyl ammonium chloride (5.0 g) and 30% sodium cyanide solution (70.0 g) were added to a 500 mL three-necked flask, and the system was stirred at room temperature for 30 min. O-chlorobenzaldehyde (50.0 g) was added to the system. After the addition was complete, the system was slowly warmed up to 30° C. for 6 h. After the reaction was completed, ammonium bicarbonate (79.0 g) was added to the system. After the addition was complete, the system continued to react at 60° C. for 6 h.
[0051] After the reaction is finished, add 30% liquid caustic soda to the system to adjust the pH value of the system to ≥12. Then the reaction solution was transferred into a self-boosting high-pressure reactor, the temperature of the system was raised to 135° C., the pressure was 0.3 MPa, and the reaction was carried out for 5 hours.
[0052] After the reaction, the temperature of the system was lowered to 70° C., activated carbon (1 g) was added, and the mixture ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com