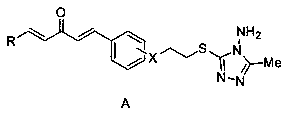
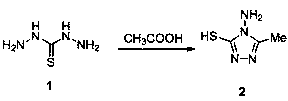1, 4-pentadiene-3-one derivative containing thioether triazole, preparation method and application
A technology of pentadiene and its derivatives, applied in the field of antiviral and antibacterial activities, and the preparation of 1,4-pentadien-3-one derivatives, to achieve excellent activity and good activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1-(4-(2-((4-Amino-5-methyl-4H-1,2,4-triazol-3-yl)thio)ethoxy)phenyl)-5-(4- The preparation method of nitrophenyl)-1,4-pentadien-3-one (target compound A1), comprises the following steps:
[0032] (1) Preparation of hydrazinothiohydrazide (intermediate 1):
[0033] Add 20mL of 85% hydrazine hydrate and 60mL of water to a three-neck flask equipped with a thermometer, dropping funnel and condenser (the upper port is connected with a tail gas outlet pipe), and control the temperature at about 50°C. Under electromagnetic stirring, 6 mL of CS2 was added dropwise within 1 h, and then the mixed solution was refluxed at 90° C. for 1 h, cooled and crystallized, filtered with suction, and recrystallized in water to obtain colorless needle-shaped crystals (intermediate 1), yield: 82% .
[0034] (2) Preparation of 4-amino-5-methyl-4H-1,2,4-triazole-3-mercapto (intermediate 2):
[0035] Take 3.18g (0.03mol) of hydrazinothiohydrazide (intermediate 1) in a flask, add 10mL of glacial...
Embodiment 2
[0043] (4-(2-((4-amino-5-methyl-4H-1,2,4-triazol-3-yl)thio)ethoxy)phenyl)-5-(4-chlorobenzene Base)-1,4-pentadien-3-ketone (target compound A2) preparation method, comprises the following steps:
[0044] (1) Preparation of hydrazinothiohydrazide (intermediate 1):
[0045] As in the first (1) step of Example 1.
[0046] (2) Preparation of 4-amino-5-methyl-4H-1,2,4-triazole-3-mercapto (intermediate 2):
[0047] As in embodiment 1 (2) step.
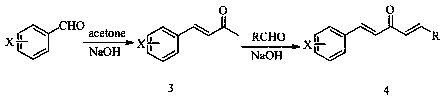
[0048] (3) Preparation of 4-(hydroxyphenyl)-3-buten-2-one (intermediate 3):
[0049] As in embodiment 1 (3) step.
[0050] (4) Preparation of 1-(4-chloro)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one:
[0051] As in step (4) of Example 1, the difference is that R is 4-chlorobenzaldehyde.
[0052] (5) Preparation of 1-(4-(2-bromoethoxy)phenyl)-5-(4-chlorophenyl)-1,4-pentadien-3-one:
[0053] As in step (5) of Example 1, the difference is that R is 4-chlorobenzaldehyde.
[0054] (6) 1-(4-(2-((4-amino-5-methyl-4H-1,2,4-triazol-3-yl)thio)ethox...
Embodiment 3
[0057] 1-(4-(2-((4-Amino-5-methyl-4H-1,2,4-triazol-3-yl)thio)ethoxy)phenyl)-5-(4- Fluorophenyl)-1,4-pentadien-3-one (target compound A3) preparation method, comprises the following steps:
[0058] (1) Preparation of hydrazinothiohydrazide (intermediate 1):
[0059] As in the first (1) step of Example 1.
[0060] (2) Preparation of 4-amino-5-methyl-4H-1,2,4-triazole-3-mercapto (intermediate 2):
[0061] As in embodiment 1 (2) step.
[0062] (3) Preparation of 4-(hydroxyphenyl)-3-buten-2-one (intermediate 3):
[0063] As in embodiment 1 (3) step.
[0064] (4) Preparation of 1-(4-fluoro)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one:
[0065] As in step (4) of Example 1, the difference is that R is 4-fluorobenzaldehyde.
[0066] (5) Preparation of 1-(4-(2-bromoethoxy)phenyl)-5-(4-fluorophenyl)-1,4-pentadien-3-one:
[0067] As in step (5) of Example 1, the difference is that R is 4-fluorobenzaldehyde.
[0068] (6) 1-(4-(2-((4-amino-5-methyl-4H-1,2,4-triazol-3-yl)thio)ethoxy)phen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com