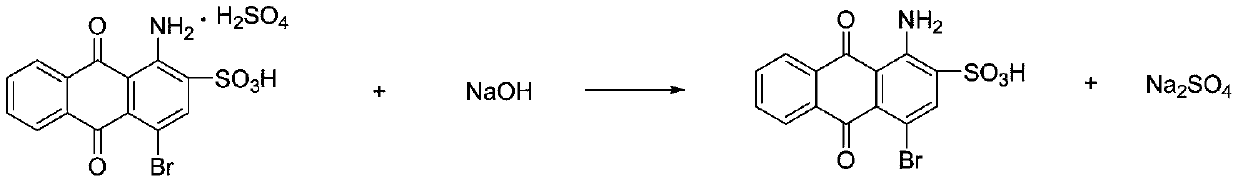
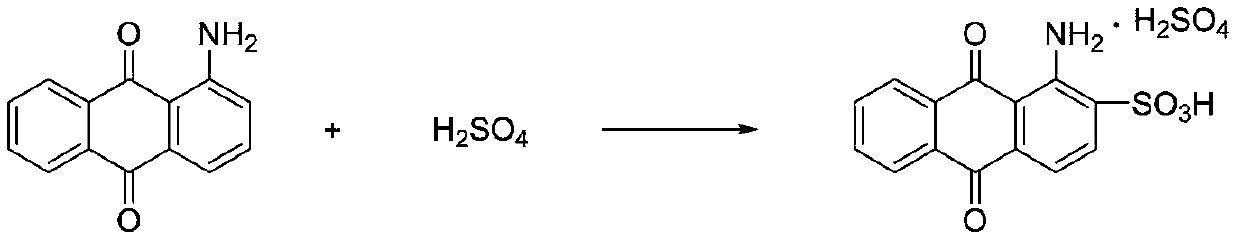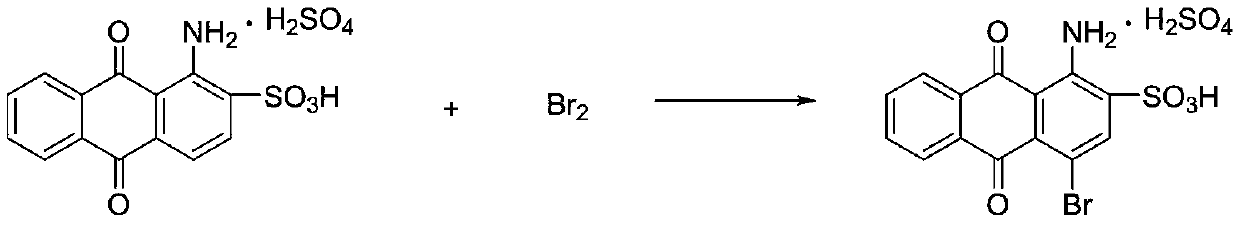Preparation method for bromamine acid
A technology of bromic acid and sodium sulfate, which is applied in the field of medicine and chemical industry, can solve the problems of unsuitability for industrial production, harsh reaction conditions, complicated process flow, etc., and achieve the effects of low production cost, mild reaction conditions and high total reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A preparation method for bromic acid, comprising the following steps:
[0038] Add 200ml of 20% oleum and 100g of anhydrous sodium sulfate into a three-necked flask with stirring, start stirring, weigh 150g of 1-aminoanthraquinone, and 750g of o-dichlorobenzene, slowly add them to the three-necked flask, heat up to 150°C and keep warm 3h; Cool the reaction solution to 80°C, add 0.2g of iodine and 0.5ml of anti-foaming agent, and slowly add 21ml of bromine dropwise within 6h, keep the temperature for 2h after dripping; remove excess bromine by distillation under reduced pressure, add 150ml of bromine to the reaction solution Water, cooled to 50°C, suction filtered to obtain 668g of filter cake; add the filter cake to 1500ml water and heat up to 90°C, and add 50% sodium hydroxide solution to adjust pH = 8, cool to 30°C, and suction filter to obtain the product bromine 238.4g, the yield is 92%, and the purity detected by liquid chromatography is 99%.
Embodiment 2
[0040] A preparation method for bromic acid, comprising the following steps:
[0041] Add 200ml of 20% oleum and 100g of anhydrous sodium sulfate into a three-necked flask with stirring, start stirring, weigh 150g of 1-aminoanthraquinone and 750g of o-dichlorobenzene and slowly add them into the three-necked flask, heat up to 100°C and keep warm 10h; cool the reaction solution to 80°C, add 0.2g of iodine and 0.5ml of anti-foaming agent, and slowly add 21ml of bromine dropwise within 6h, and keep warm for 2h after dropping; remove excess bromine by distillation under reduced pressure, and add 150ml of bromine to the reaction solution Water, cooled to 50°C, suction filtered to obtain 668g of filter cake; add the filter cake to 1500ml water and heat up to 90°C, and add 50% sodium hydroxide solution to adjust pH = 8, cool to 30°C, and suction filter to obtain the product bromine 238.4g, the yield is 92%, and the purity detected by liquid chromatography is 99%.
Embodiment 3
[0043] A preparation method for bromic acid, comprising the following steps:
[0044] Add 200ml of 20% oleum and 100g of anhydrous sodium sulfate into a three-necked flask with stirring, start stirring, weigh 150g of 1-aminoanthraquinone, and 750g of o-dichlorobenzene, slowly add them into the three-necked flask, heat up to 130°C and keep warm 5h; cool the reaction solution to 80°C, add 0.2g of iodine and 0.5ml of anti-foaming agent, and slowly add 21ml of bromine dropwise within 5h. Water, cooled to 50°C, suction filtered to obtain 668g of filter cake; add the filter cake to 1500ml water and heat up to 90°C, and add 50% sodium hydroxide solution to adjust pH = 8, cool to 30°C, and suction filter to obtain the product bromine 238.4g, the yield is 92%, and the purity detected by liquid chromatography is 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com