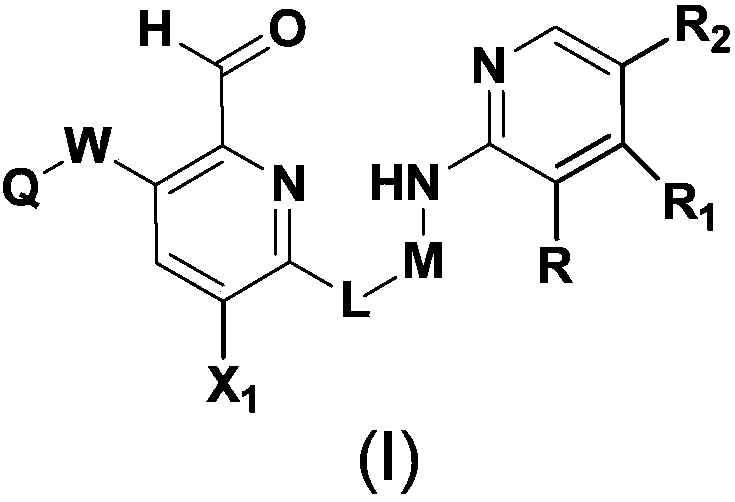
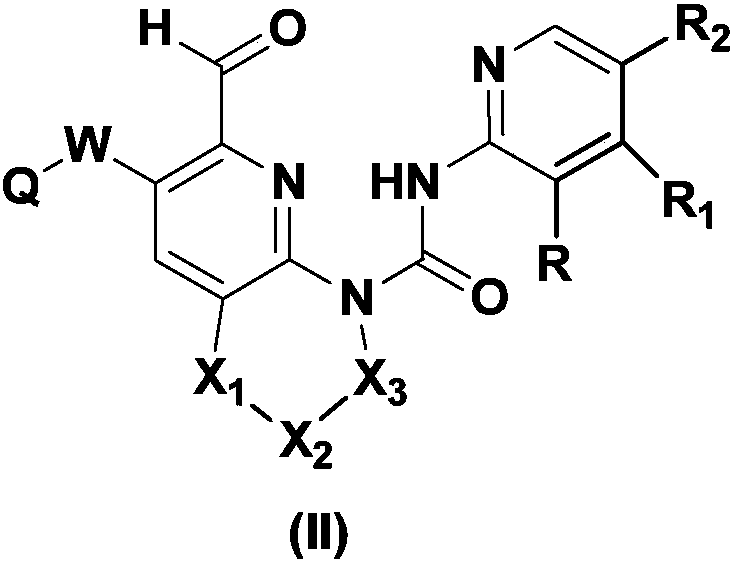
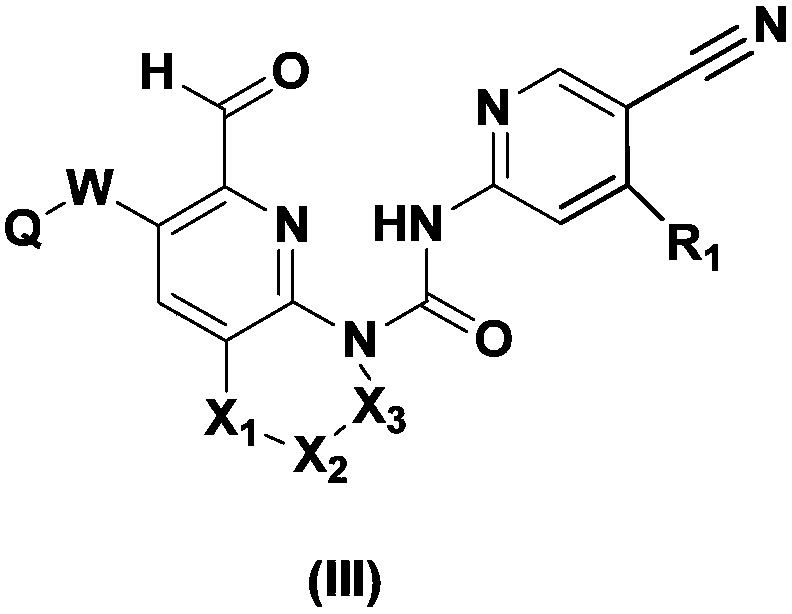
Pharmaceutical composition containing FGFR4 inhibitor
A composition and drug technology, applied in the field of drugs, can solve the problems of side effects, weak inhibition of FGFR4 activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0337] Preparation of intermediates
[0338] Intermediate 1: Preparation of 6-amino-4-fluoronicotine nitrile
[0339]
[0340] The first step: the preparation of 4-fluoro-5-iodopyridin-2-amine
[0341]
[0342] 4-fluoropyridin-2-amine (9g, 80mmol), NIS (19.8g, 88mmol), TFA (3.65g, 32mmol) were mixed in MeCN (290mL), and reacted overnight at room temperature. Diluted with ethyl acetate (300 mL), saturated with Na 2 SO 3 The aqueous solution (150 mL×2) was washed, the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to give the title compound 4-fluoro-5-iodopyridin-2-amine (15.8 g, 83%).
[0343] MS m / z(ESI):238.9[M+H] + .
[0344] The second step: the preparation of 6-amino-4-fluoronicotine nitrile
[0345]
[0346] 4-Fluoro-5-iodopyridin-2-amine (15.8g, 66.4mmol), Zn(CN) 2 (8.2g, 69.8mmol), Zn (0.87g, 13.3mmol) were mixed in DMA (55mL), and Pd was added under nitrogen atmosphere 2 (dba) 3 (2.4g, 2.62mmol) and...
Embodiment 1
[0694] N-(5-cyano-4-(((trans)-2-methoxycyclopentyl)amino)pyridin-2-yl)-7-formyl-6-((3-carbonylmorpholino )methyl)-3,4-dihydro-1,8-naphthyridine-1(2H)-carboxamide
[0695]
[0696] The first step: N-(5-cyano-4-(((trans)-2-methoxycyclopentyl)amino)pyridin-2-yl)-7-(dimethoxymethyl)- Preparation of 6-((3-carbonylmorpholino)methyl)-3,4-dihydro-1,8-naphthyridine-1(2H)-carboxamide
[0697]
[0698] 6-amino-4-(((trans)-2-methoxycyclopentyl)amino)nicotine nitrile (20mg, 0.09mmol), phenyl 7-(dimethoxymethyl)-6-(( 3-Carbonylmorpholino)methyl)-3,4-dihydro-1,8-naphthyridine-1(2H)-carboxylate (38 mg, 0.09 mmol) was mixed in THF (5 mL), N 2 Under the atmosphere, it was cooled to -78°C, a solution of LiHMDS in THF (0.2 mL, 0.2 mmol) was added dropwise, and the reaction was allowed to rise to room temperature overnight. Add saturated NH 4 Cl aqueous solution (50mL), extracted with ethyl acetate (50mL×2), the organic phase was washed with saturated brine, dried over anhydrous sodium s...
Embodiment 29
[0706] (R)-N-(5-cyano-4-((1-methoxypropan-2-yl)amino)pyridin-2-yl)-7-formyl-6-((2-carbonyl-1 ,3-oxazepan-3-yl)methyl)-3,4-dihydro-1,8-naphthyridine-1(2H)-carboxamide
[0707]
[0708] The first step: (R)-N-(5-cyano-4-((1-methoxypropan-2-yl)amino)pyridin-2-yl)-7-(dimethoxymethyl) -6-((2-carbonyl-1,3-oxazepan-3-yl)methyl)-3,4-dihydro-1,8-naphthyridine-1(2H)-carboxamide synthesis
[0709]
[0710](R)-6-amino-4-((1-methoxypropan-2-yl)amino)nicotine nitrile (30mg, 0.14mmol), phenyl 7-(dimethoxymethyl)-6-( (2-Carbonyl-1,3-oxazepan-3-yl)methyl)-3,4-dihydro-1,8-naphthalene-1(2H)-carboxylate (60mg, 0.13 mmol) was dissolved in THF (5mL), N 2 Cool to -78°C under atmosphere, add a solution of LiHMDS in THF (0.3mL, 0.3mmol) dropwise to the reaction solution, and let it rise to room temperature to react overnight. Add saturated NH 4 Cl aqueous solution (50mL), extracted with ethyl acetate (50mL×2), the combined organic phases were washed with saturated brine, dried over anhydrou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com