Organic porous polymer palladium-supported catalyst and preparation and application methods thereof
A technology of porous polymer and palladium catalyst is applied in the preparation of organic compounds, organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, etc. Industrial applications, catalysts are not easy to separate, etc., to achieve good application prospects and economic value, good recycling performance, and small loss of palladium.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: Preparation of organic porous polymer supported palladium catalyst (Pd@POP-1)
[0051] 4,5-bisdiphenylphosphine-9,9-dimethylxanthene palladium dichloride (0.005mol), triphenylbenzene (0.005mol), dimethylformal (0.04mol) and 20ml The 1,2-dichloroethane solution was mixed evenly, and then the anhydrous FeCl 3 (0.04mol) was added to the above solution and stirred evenly to obtain a reaction solution. in N 2 Under protection, the reaction solution was heated up to 45°C, stirred at 45°C for 3 hours for pre-polymerization, then heated to 80°C, stirred for 24 hours at 80°C for polymerization. After the reaction, the obtained mixture was filtered, and the obtained precipitate was washed 6 times with anhydrous methanol, and then vacuum-dried at 60° C. for 12 hours to obtain 5.91 g of organic porous polymer-supported palladium catalyst, which was named Pd@POP-1.
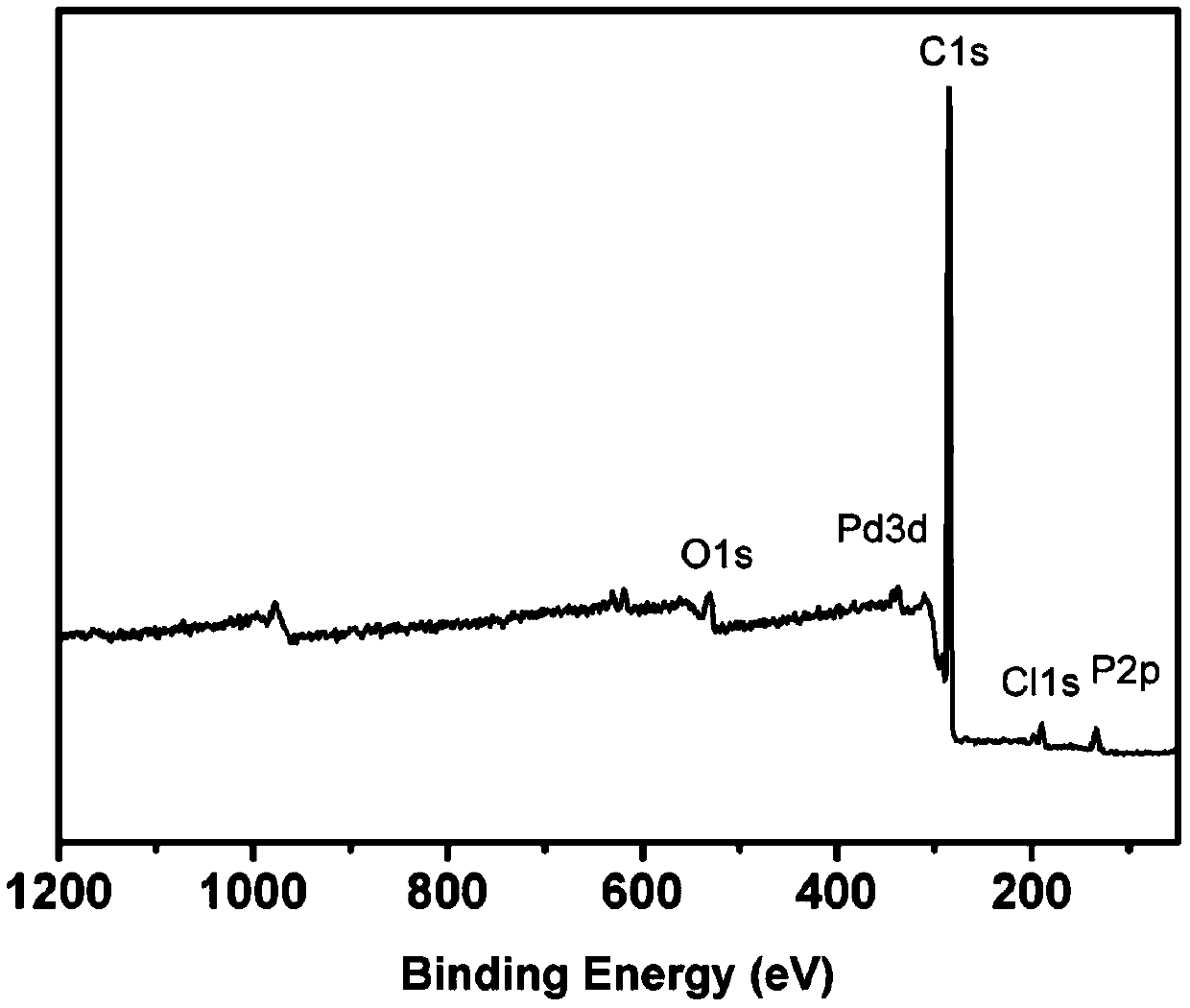
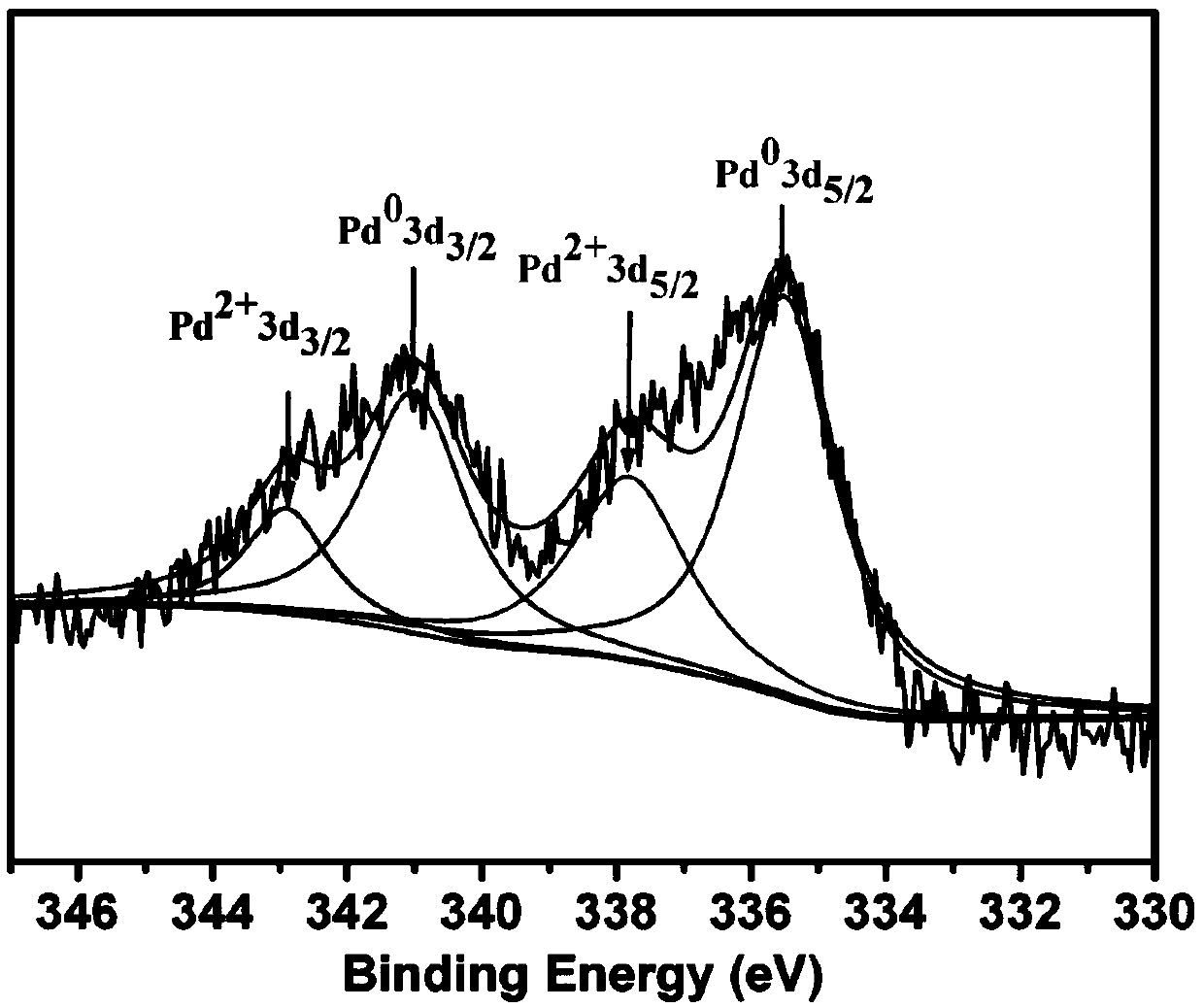
[0052] Pd@POP-1 was analyzed by X-ray photoelectron spectrometer, and the spectrum is as follows fi...
Embodiment 2
[0057] Embodiment 2: Preparation of organic porous polymer supported palladium catalyst (Pd@POP-2)
[0058] This example is operated according to the preparation method of Example 1, the only difference is that the raw material 4,5-bisdiphenylphosphine-9,9-dimethylxanthene palladium dichloride is 0.003mol, triphenyl The base benzene is 0.007mol, and the other operations are exactly the same as in Example 1, and finally 5.05g of Pd@POP-2 is obtained.
[0059] As in Example 1, the prepared Pd@POP-2 was subjected to the following characterization analysis:
[0060] The Pd@POP-2 was analyzed by X-ray photoelectron spectroscopy, and the results showed that the prepared C, P, Pd, O, Cl existed, and palladium had divalent palladium (Pd 2+ ) and zero price (Pd 0 ) 2 valence states.
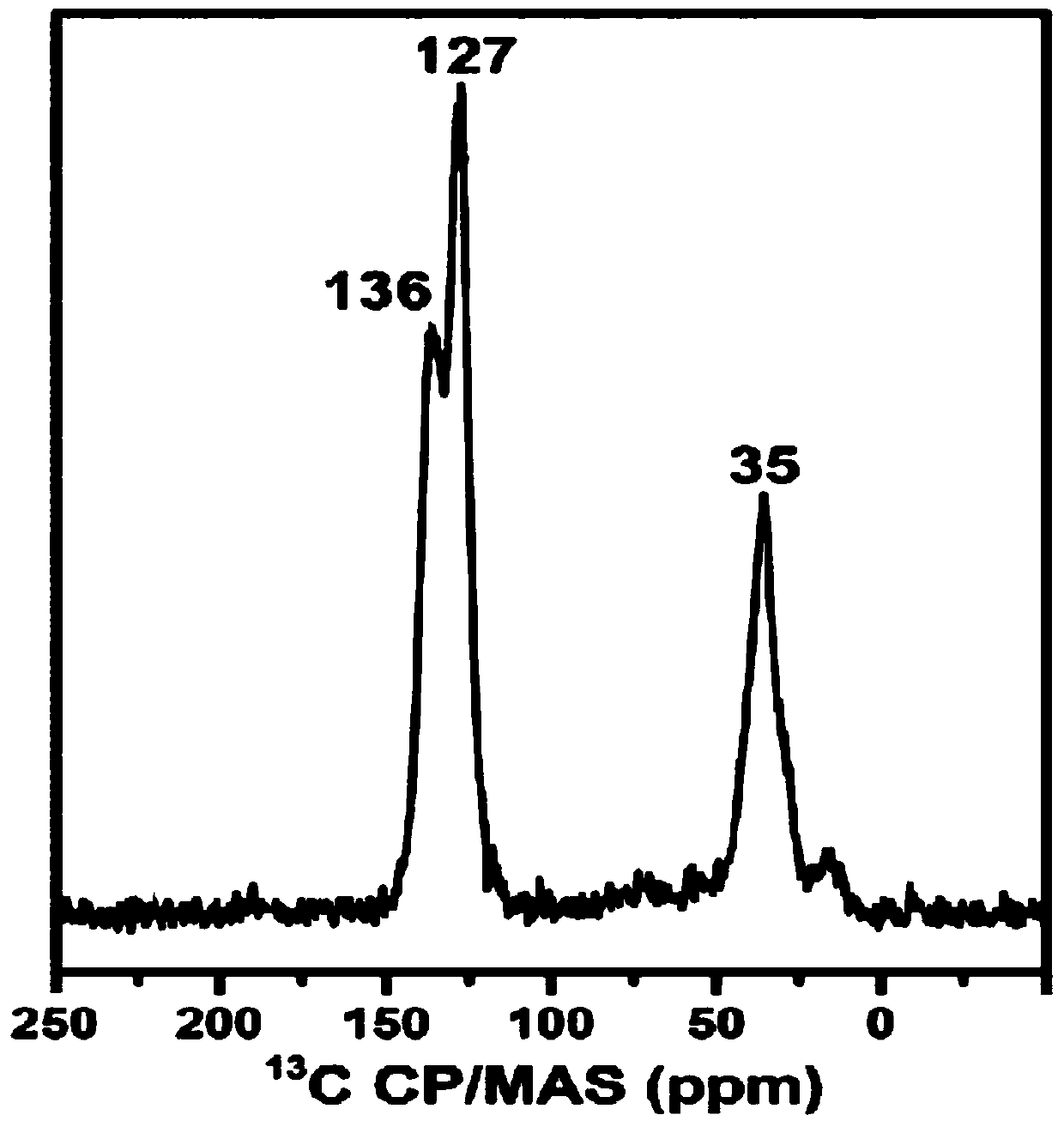
[0061] Using cross-polarized magic-angle spinning solid-state NMR- 13 C Spectrum ( 13 C-CP / MAS) performed characterization analysis on Pd@POP-2, the characteristic peak of substituted benzene ring ca...
Embodiment 3
[0065] Embodiment 3: Preparation of organic porous polymer supported palladium catalyst (Pd@POP-3)
[0066] This example is operated according to the preparation method of Example 1, the only difference is that the solvent used is chloroform (20ml), other operations are exactly the same as Example 1, and finally 5.86g of Pd@POP-3 is obtained.
[0067] As in Example 1, the prepared Pd@POP-3 was subjected to the following characterization analysis:
[0068] The Pd@POP-3 was analyzed by X-ray photoelectron spectroscopy, and the results showed that the prepared C, P, Pd, O, Cl existed, and palladium had divalent palladium (Pd 2+ ) and zero price (Pd 0 ) 2 valence states.
[0069] Using cross-polarized magic-angle spinning solid-state NMR- 13 C Spectrum ( 13 C-CP / MAS) performed characterization analysis on Pd@POP-3, the characteristic peak of substituted benzene ring carbon appeared at 136ppm, the characteristic peak of unsubstituted benzene ring carbon appeared at 128ppm, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com