Method for synthesizing 2-(5-fluoro-2, 4 dinitro-phenoxy) acetate
A technology of dinitrophenoxy and dinitrobenzene, which is applied in the field of synthesizing 2-acetate, can solve the problems of severe nitration reaction conditions, easy breakage of ether bonds, and low product yield, so as to improve fluorination activity, High conversion rate and strong fluorination selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] The following will clearly and completely describe the technical solutions in the embodiments of the present invention with reference to the accompanying drawings in the embodiments of the present invention. Obviously, the described embodiments are only some, not all, embodiments of the present invention. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts belong to the protection scope of the present invention.
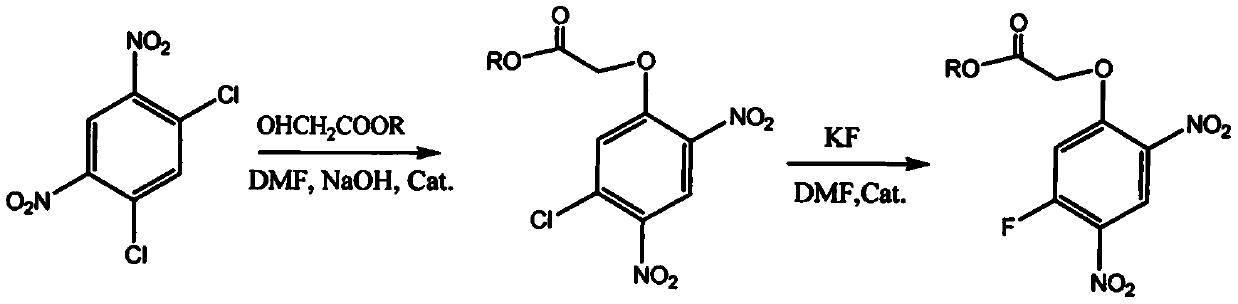
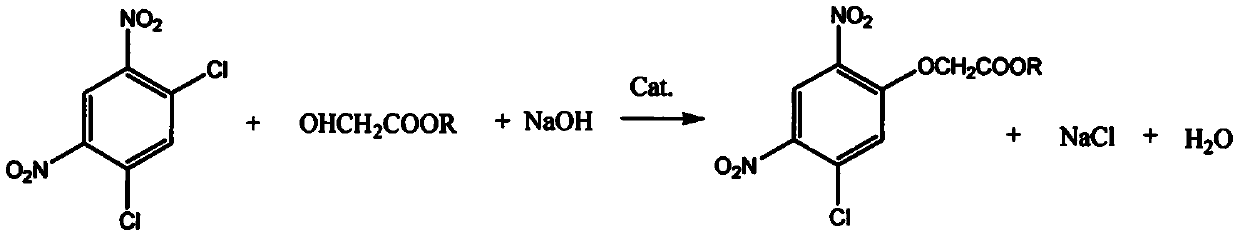
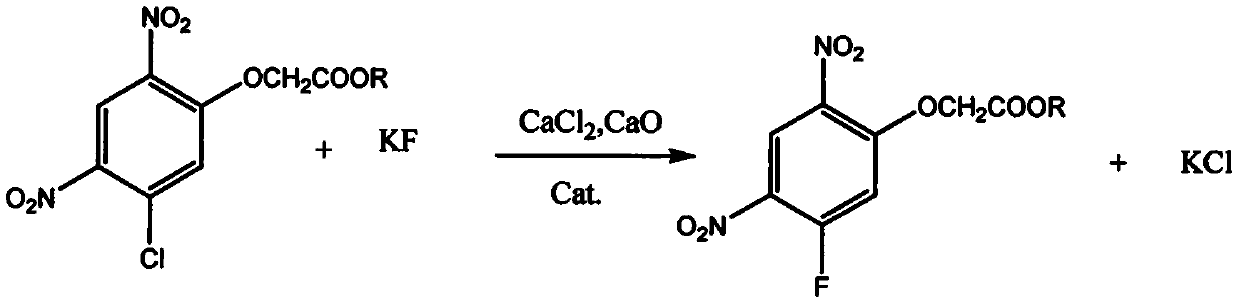
[0018] see figure 1 , a method for synthesizing 2-(5-fluoro-2,4-dinitrophenoxy) acetate, using 2,4-dichloro-1,5-dinitrobenzene as raw material through etherification and fluorination Reaction and workup give 2-(5-fluoro-2,4-dinitrophenoxy)acetate.
[0019] A further technical solution of the present invention comprises the following steps:
[0020] (1) etherification reaction
[0021] Put 500ml of DMF into a 1000ml reaction bottle, add 300g of 2,4-dichloro-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com