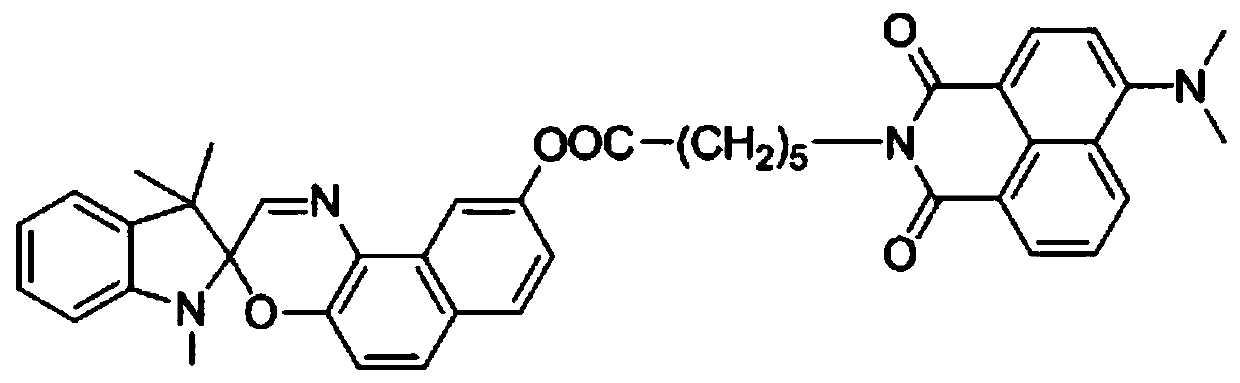
Spirooxazine type hydrogen ion fluorescent probe compound based on 1,8-naphthalimide unit, and synthesis method and application of compound
A naphthalimide and fluorescent probe technology, which is applied in chemical instruments and methods, fluorescence/phosphorescence, color-changing fluorescent materials, etc., to achieve the effect of simplifying the purification steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
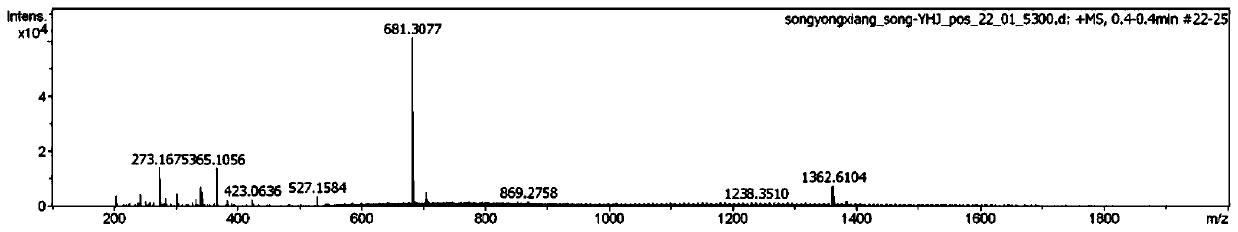
Image
Examples
Embodiment
[0039] The concrete synthetic route of present embodiment compound comprises the following steps:
[0040] Synthesis of S1, 1-nitroso-2,7-binaphthol 1
[0041] Dissolve 4g of 2,7-binaphthol in 80mL of near-boiling distilled water, stir evenly, and quickly pour it into a three-necked bottle equipped with an electric stirrer, add an aqueous solution of sodium nitrite (1.84g of nitrous acid Sodium dissolved in 40mL distilled water), after mixing well, slowly add sulfuric acid solution dropwise (mix 1.44g concentrated sulfuric acid with 20mL water and cool), control the temperature below 0°C, continue stirring for 1h after the dropwise addition, add a small amount of ammonium sulfate to remove Excess nitrous acid was filtered under reduced pressure after the reaction was complete, the solid was washed with distilled water and then vacuum-dried to obtain 4.20 g of purple-red compound 1.
[0042] S2. Synthesis of compound 1,3,3-trimethylspiroindoline-2,3′[3H]-naphtho[2,1-b][1,4]oxa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com