N-substituted imidazole formate derivative and application thereof
An unsubstituted and deuterated technology, applied in the field of medicinal chemistry, can solve problems such as not found
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
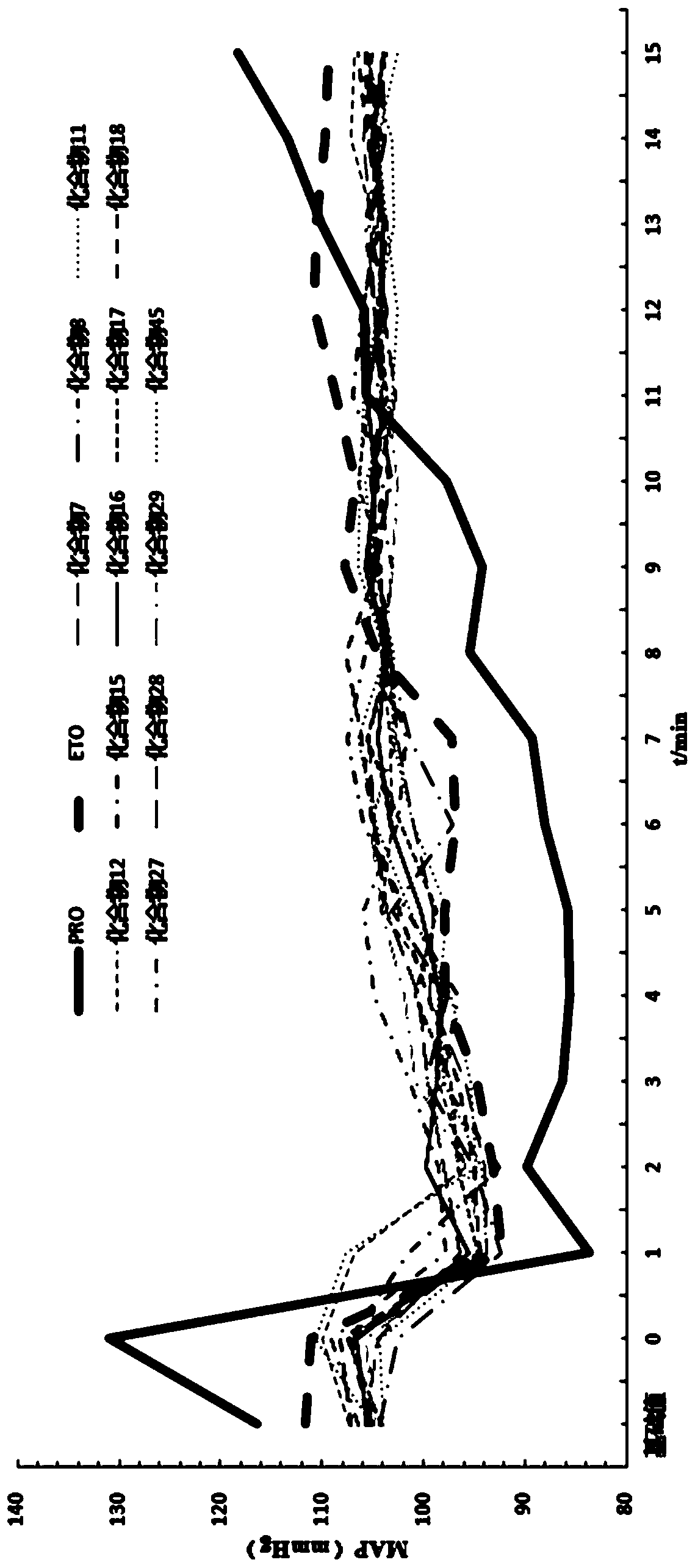
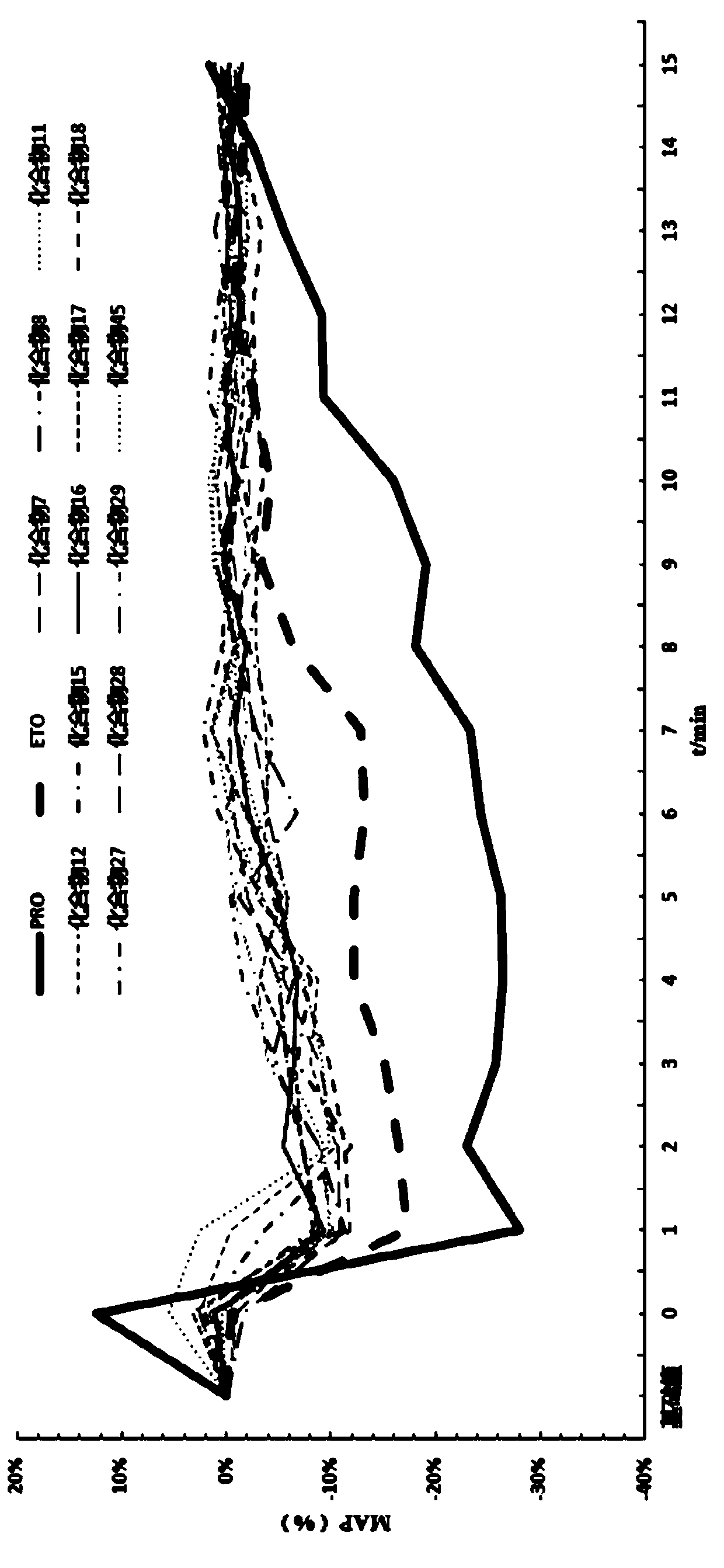
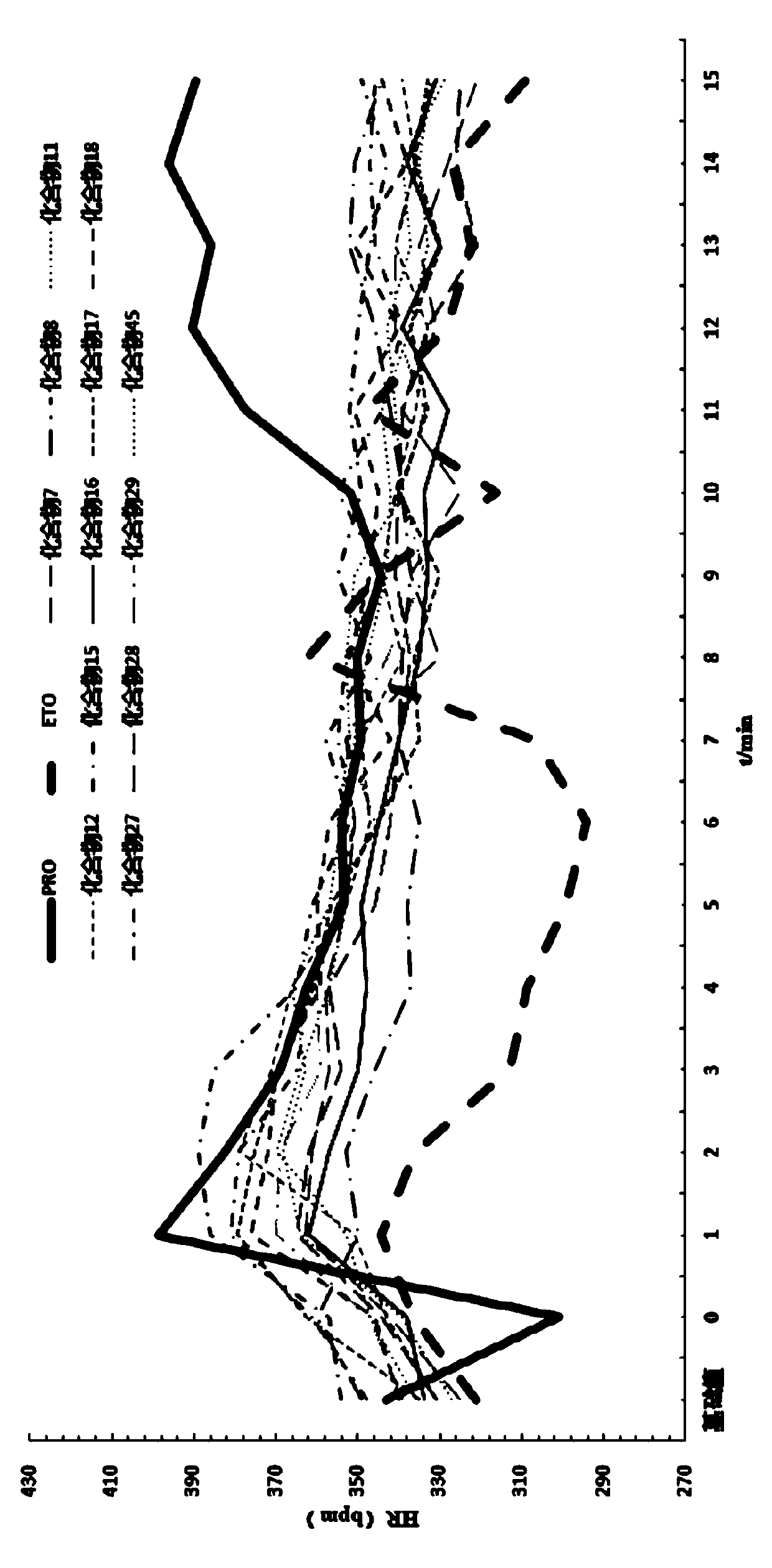
Image
Examples
Embodiment 1
[0224] Embodiment 1 Preparation of Compound 1 of the present invention
[0225]
[0226] 1, the preparation of 1-ethynyl cyclobutanol (1-2)
[0227]
[0228] Cyclobutanone (1-1) (700.6mg, 10.0mmol) was dissolved in anhydrous THF (10mL) solution, the reaction system was replaced with nitrogen, and the temperature was lowered to 0°C with an ice-water bath. At 0°C, propargylmagnesium bromide (26 mL, 1.3 mol / L inTHF, 13 mmol) was slowly added to the system over 25 minutes with a syringe, and reacted at room temperature for two hours. The complete reaction was monitored by TLC, the reaction system was terminated with saturated ammonium chloride solution (30mL), extracted with dichloromethane (3×10mL), the combined organic phases were washed with saturated brine (50mL), dried over anhydrous sodium sulfate, and extracted Filtered and concentrated under reduced pressure to obtain colorless oily crude product 1-2, which was directly used in the next reaction without further puri...
Embodiment 2
[0233] Embodiment 2 Preparation of Compound 2 of the present invention
[0234]
[0235] 1. Preparation of 3-(1-Ethoxyvinyl)oxetan-3-ol(2-2)
[0236]
[0237] Dissolve vinyl ethyl ether (2-1) (3.60g, 50.0mmol) in anhydrous THF (20mL), replace the reaction system with nitrogen, and cool down to -78°C with dry ice acetone under the protection of nitrogen. Lithium (20 mL, 1.0 mol / L inpentane, 20 mmol) was slowly added to the system within 5 minutes, and the temperature was kept below -70°C during the addition. After adding tert-butyllithium, continue to react at -78°C for 15 minutes, then replace the dry ice acetone bath with an ice water bath, continue the reaction for 15 minutes, then use a dry ice acetone bath to lower the temperature to -78°C, and place the 3-oxoheterocyclic Butanone (720.6 mg, 10.0 mmol) in anhydrous THF (20 mL) was slowly added to the reaction system over 10 minutes. The reaction system was naturally warmed to room temperature, and the reaction was ...
Embodiment 3
[0242] Embodiment 3 Preparation of Compound 3 of the present invention
[0243]
[0244] 1. Preparation of 3-Ethynyloxetan-3-ol (3-2)
[0245]
[0246]Dissolve vinyl ethyl ether (3-1) (3.60g, 50.0mmol) in anhydrous THF (20mL), replace the reaction system with nitrogen, and cool down to -78°C with dry ice acetone under the protection of nitrogen. Lithium (20 mL, 1.0 mol / L inpentane, 20 mmol) was slowly added to the system within 5 minutes, and the temperature was kept below -70°C during the addition. After adding tert-butyllithium, continue to react at -78°C for 15 minutes, then replace the dry ice acetone bath with an ice water bath, continue the reaction for 15 minutes, then use a dry ice acetone bath to lower the temperature to -78°C, and place the 3-oxoheterocyclic Butanone (720.6 mg, 10.0 mmol) in anhydrous THF (20 mL) was slowly added to the reaction system over 10 minutes. The reaction system was naturally warmed to room temperature, and the reaction was continue...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com