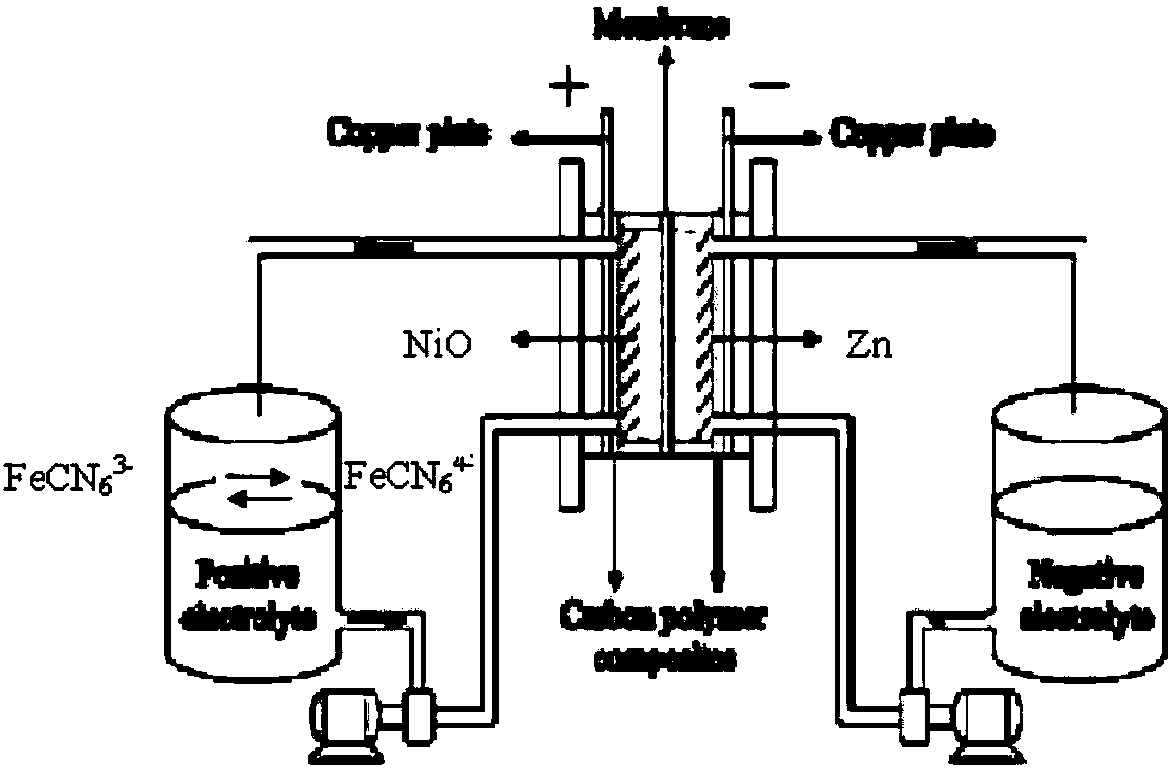
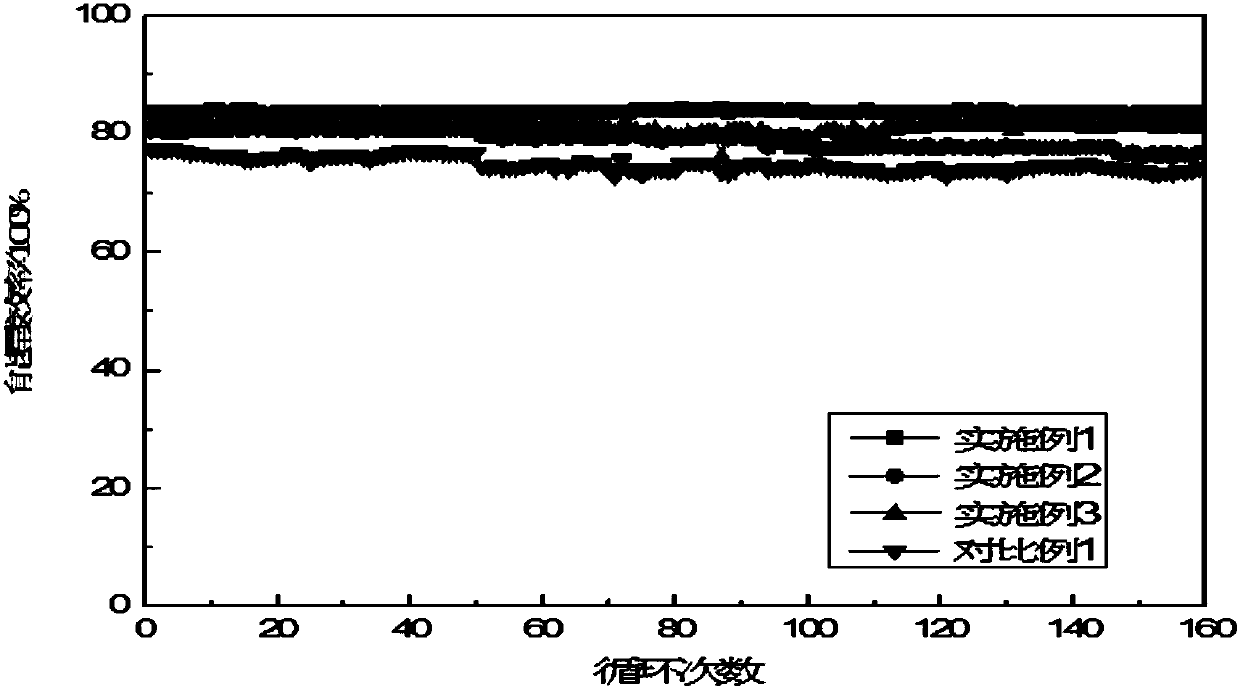
Hybrid zinc-nickel redox flow battery
A flow battery and hybrid technology, which can be used in regenerative fuel cells and other directions, can solve the problems of low solid-phase electrode surface capacity and difficulty in meeting the capacity requirements of large-scale energy storage batteries.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Hybrid zinc-nickel flow battery:
[0022] 1) The positive electrode uses a 5*5cm NiO electrode;
[0023] 2) The negative electrode uses 5*5cm carbon felt (5mm thickness) and 5*5cm zinc sheet (1mm thickness);
[0024] 3) Use 30 mL of 0.2 mol / L potassium ferrocyanide in 3 mol / L potassium hydroxide solution as the positive electrode electrolyte;
[0025] 4) Negative electrode electrolyte adopts 3mol / L potassium hydroxide solution of 0.5mol / L zinc ion of 30mL;
[0026] 5) The diaphragm adopts cationic membrane Nafion115;
[0027] 6) Battery at 40mA / cm 2 Under charge and discharge, charging time: 1h, discharge cut-off voltage: 1.0V.
Embodiment 2
[0029] Hybrid zinc-nickel flow battery:
[0030] 1) The positive electrode uses a 5*5cm NiO electrode;
[0031] 2) The negative electrode uses 5*5cm carbon felt (5mm thickness) and 5*5cm zinc sheet (1mm thickness);
[0032] 3) The positive electrode electrolyte is 30 mL of 8 mol / L potassium hydroxide solution of 0.5 mol / L potassium ferrocyanide;
[0033] 4) Negative electrode electrolyte adopts 8mol / L potassium hydroxide solution of 0.8mol / L zinc ion of 30mL;
[0034] 5) The diaphragm adopts cationic membrane Nafion115;
[0035] 6) Battery at 40mA / cm 2 Under charge and discharge, charging time: 1h, discharge cut-off voltage: 1.0V.
Embodiment 3
[0037] Hybrid zinc-nickel flow battery:
[0038] 1) The positive electrode uses a 5*5cm NiO electrode;
[0039] 2) The negative electrode uses 5*5cm carbon felt (5mm thickness) and 5*5cm zinc sheet (1mm thickness);
[0040] 3) The positive electrode electrolyte adopts 30mL of 6mol / L potassium hydroxide solution of 1mol / L potassium ferrocyanide;
[0041] 4) Negative electrode electrolyte adopts 6mol / L potassium hydroxide solution of 0.1mol / L zinc ion of 30mL;
[0042] 5) The diaphragm adopts cationic membrane Nafion115;
[0043] 6) Battery at 40mA / cm 2 Under charge and discharge, charging time: 1h, discharge cut-off voltage: 1.0V.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com