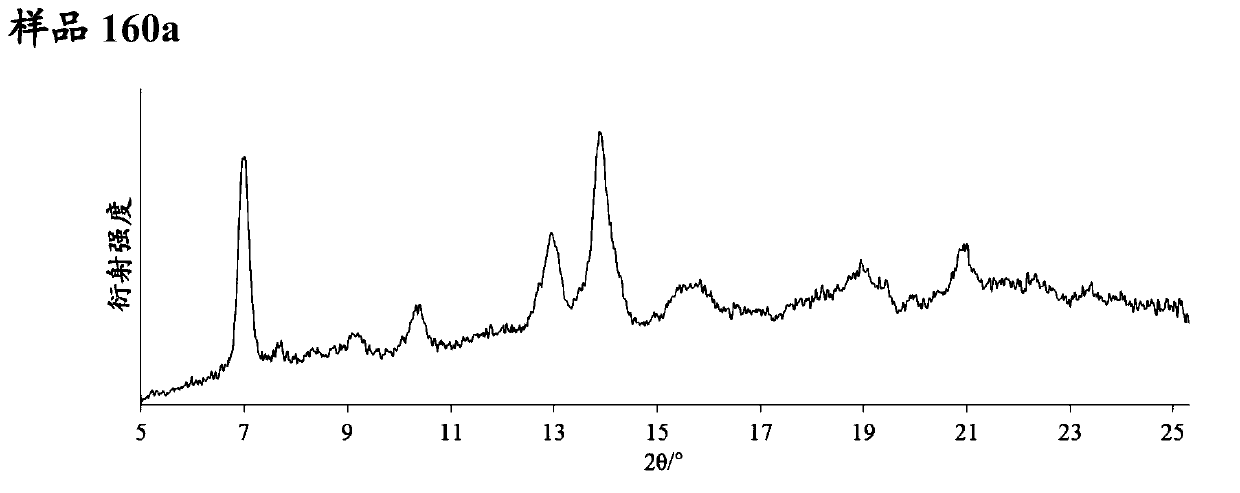
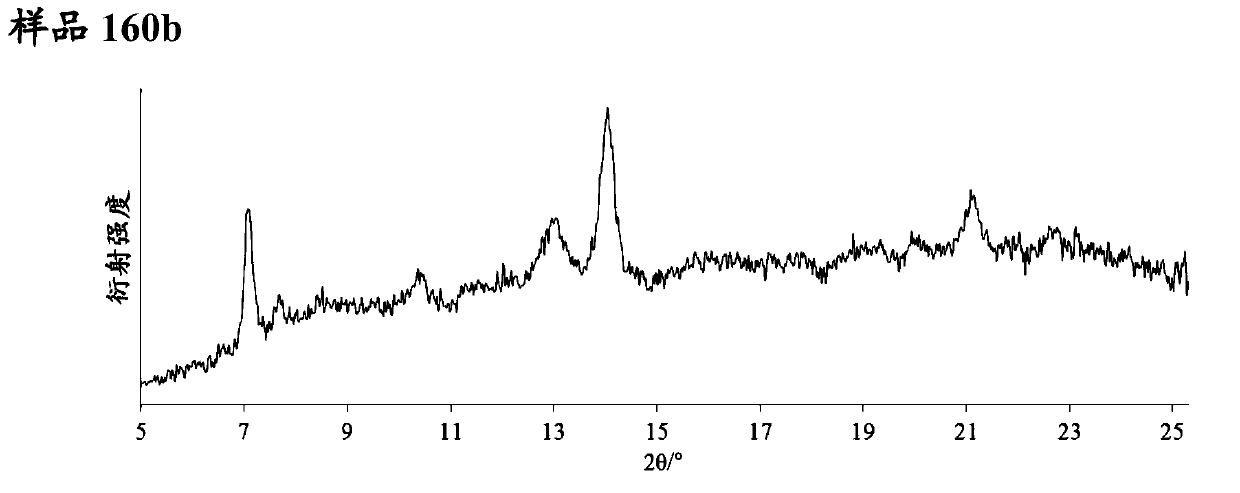
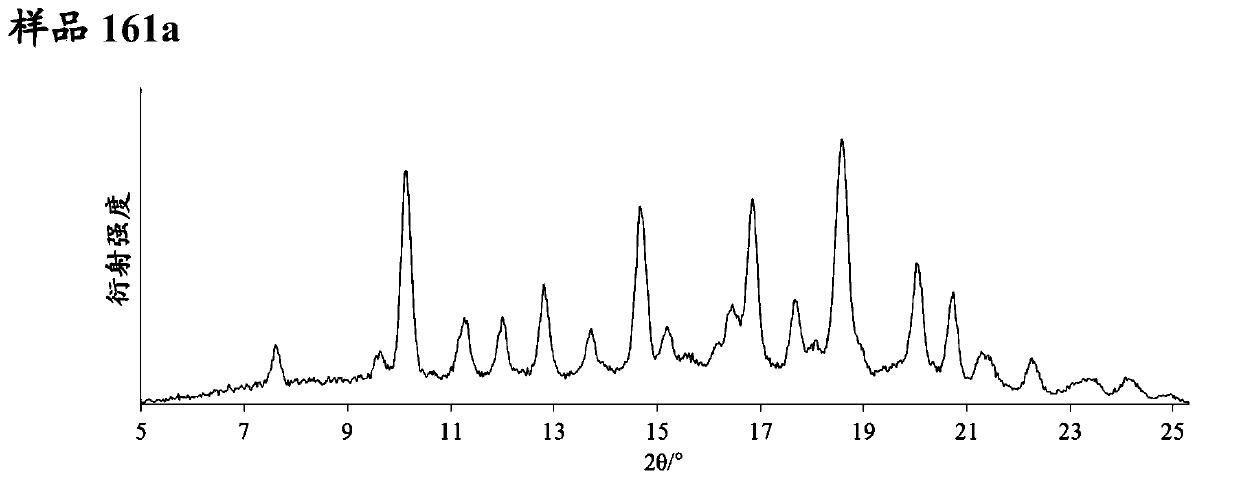
Pyrazolopyridine derivative having glp-1 receptor agonist effect
A technology of compound and alkyl, applied in the direction of medical preparations containing active ingredients, drug combination, organic chemistry, etc., can solve the problem of invasive subcutaneous administration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0407] 3-[(1S,2S)-1-[2-[2-(3,5-dimethylphenyl)-3-[3-(1-methylindazol-5-yl) )- 2-oxoimidazol-1-yl]-6,7-dihydro-4H-pyrazolo[4,3-c]pyridine-5-carbonyl]-5-(2-ethyl-3-methylpyridine - 4-yl)indol-1-yl]-2-methylcyclopropyl]-4H-1,2, 4 - Synthesis of Oxadiazol-5-one (Compound 1)
[0408] [chemical formula 17]
[0409]
[0410]
[0411] [(5-cyano-1,2,3,6-tetrahydropyridin-4-yl)amide] potassium (compound 1b)
[0412] To a solution of 3-(2-cyanoethylamino)propionitrile (compound 1a, 22.0 g, 179 mmol) in tetrahydrofuran (THF) (179 mL) was added a 1M solution of potassium tert-butoxide in THF (179 mL), and the mixture was dissolved in Stir at room temperature for 1 hour. The reaction mixture was filtered, washed with THF (50 mL), and the filtrate was dried under reduced pressure to give the title compound 1b (23.8 g, 83% yield) as a light brown solid.
[0413] LC / MS mass spectrum: m / z 124 ([M+H] + ).
[0414] LC / MS retention time: 0.14 minutes (analysis conditions:...
Embodiment 2 to 50
[0467] Apply the combination of the 2-oxoimidazole compound shown in Table 2-2 and the halogen compound shown in the following Table 2-3, and an appropriate solvent, carry out the operation similar to Example 1 step 1-11, and pass The following reactions gave Example compounds 2 to 50 shown in Table 2-1.
[0468] [chemical formula 18]
[0469]
[0470] [table 2-1]
[0471] Table 2-1. Obtained embodiment compounds 2 to 50
[0472]
[0473]
[0474]
[0475]
[0476]
[0477]
[0478]
[0479]
[0480]
[0481]
[0482]
[0483]
[0484]
[0485]
[0486]
[0487]
[0488]
[0489]
[0490]
[0491]
[0492]
[0493]
[0494]
[0495]
[0496]
[0497] The compounds in Table 2-1 have rotamers, and as an example, the compound of Example 2 has 1 H-NMR is shown below.
[0498] Rotamer A
[0499] 1 H-NMR (600MHz, CDCl 3 )δ:11.29(1H,s),8.40(1H,d,J=5.2Hz),7.93(1H,s),7.74(1H,d,J=1.5Hz),7.70(1H,d,J=8.6 Hz...
Embodiment 51 to 53
[0951] By the following reaction, 3-[(1S,2S)-1-[5-bromo-2-[2-(4-fluoro-3,5-dimethylphenyl)-3-[3-(1 -Methylindazol-5-yl)-2-oxoimidazol-1-yl]-6,7-dihydro-4H-pyrazolo[4,3-c]pyridine-5-carbonyl]indole- 1-yl]-2-methylcyclopropyl]-4H-1,2,4- Oxadiazol-5-one (compound 51d), substituted morpholine and appropriate reagents, followed by the similar operation to Step 7-1 of Example 7, to obtain Example compounds 51 to 53 shown in Table 2-4.
[0952] [chemical formula 48]
[0953]
[0954] [Table 2-4]
[0955] Table 2-4. Obtained example compounds 51 to 53
[0956]
[0957]
[0958] Compound 51d was synthesized as follows.
[0959] [chemical formula 49]
[0960]
[0961]
[0962] 2-(4-fluoro-3,5-dimethylphenyl)-3-(2-oxo-1H-imidazol-3-yl)-6,7-dihydro-4H-pyrazolo[4, 3-c] tert-butyl pyridine-5-carboxylate (compound 51a)
[0963] Add triethylamine (0.936mL, 6.72mmol), di-tert-butyl dicarbonate (0.425mL , 1.85 mmol), and the suspension was stirred at room temperature f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com