Improved process for the production of fucosylated oligosaccharides
A technology of fucosyl and oligosaccharides, applied in biochemical equipment and methods, glycosyltransferases, oxidoreductases, etc., can solve problems such as inability to achieve HMO titer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Transformation of Escherichia coli BL21(DE3) strain for producing 2'-fucosyllactose
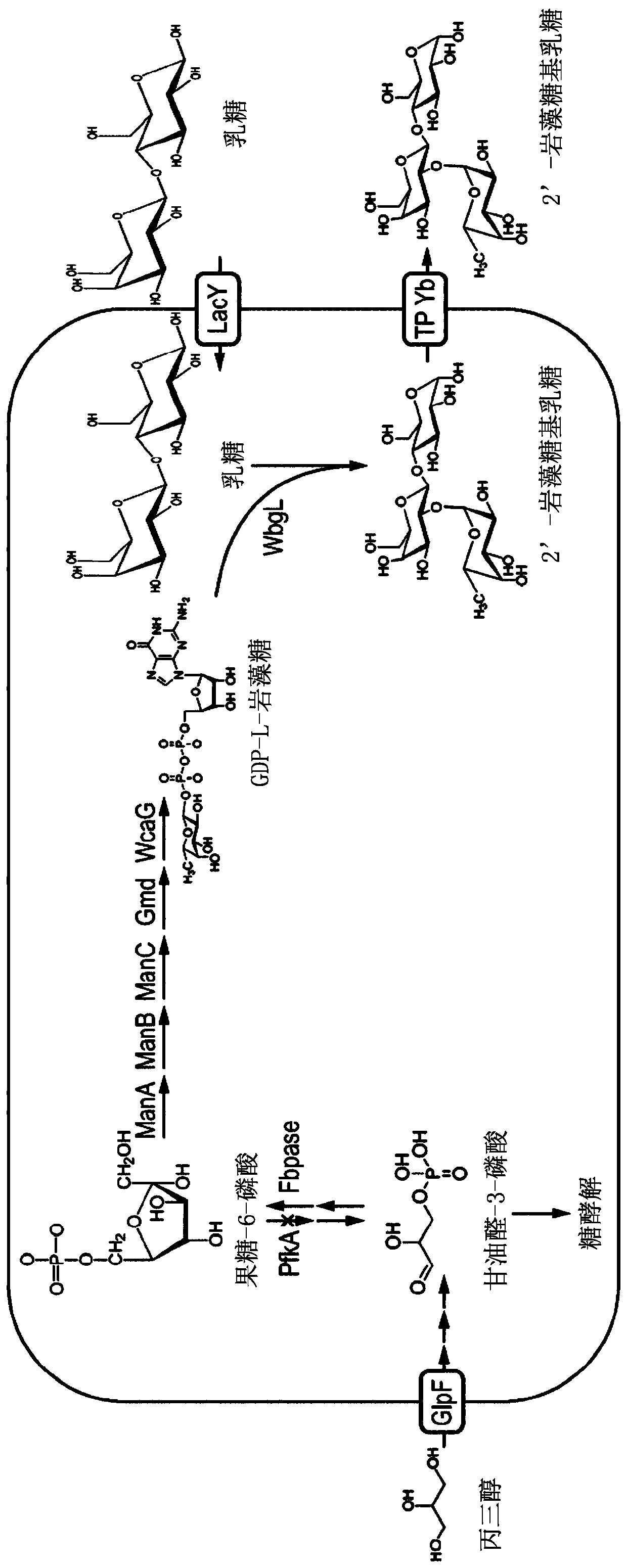
[0113] Using Escherichia coli BL21(DE3) as a parental host, a strain for producing 2'-fucosyllactose in a whole-cell biosynthesis method was constructed. Genome engineering of the strains includes gene disruption and deletion events as well as integration of heterologous genes.
[0114] Since 2'-fucosyllactose is synthesized from lactose (suitable for bacterial cultures), and from GDP-L-fucose produced in living cells, the encoding Inactivation of the wild-type copy of the lacZ gene of the endogenous β-galactosidase (see Ellis et al., "High efficiency mutagenesis, repair, and engineering of chromosome DNA using single-stranded oligonucleotides", Proc. Natl. Acad. Sci. USA 98:6742-6746 (2001)). Using the same method, the arabinose isomerase gene araA was disrupted.
[0115] The lacZΩ gene fragment was introduced under the control of the temperature-sensitive transcriptional repress...
Embodiment 2
[0121] Confirmation of increased 2'-fucosyllactose export by the Yersinia burgdorferi ATCC 43970 sugar efflux transporter
[0122] Knockout of yberc0001_9420
[0123] To demonstrate the functionality of the heterologous sugar transporter from Yersinia burgdorferi ATCC 43970, the gentamicin resistance cassette aacC1 from plasmid pBBR-MCS5 was used (Kovach, Elzer et al. 1995, "Four new derivatives of the broad-host-range cloning vector pBBRI MCS, carrying different antibiotic-resistance cassettes", Gene 166, 175-176) - which was inserted into the gene yberc0001_9420 from strain E. coli BL21(DE3) lacZ - 、araA - 、fucl - 、fucK - , wcaJ - (which contained manB, manC, gmd, wcaG, lacY, wbgL, and yberc0001_9420 chromosomal integration by homologous recombination of Datsenko and Wanner (2000; see above)) the gene yberc0001_9420 was deleted, resulting in strain Δyberc0001_9420.
[0124] Culture conditions for 2’-fucosyllactose production
[0125] The Escherichia coli BL21(DE3...
Embodiment 3
[0132] Production of 2’-fucosyllactose during fermentation
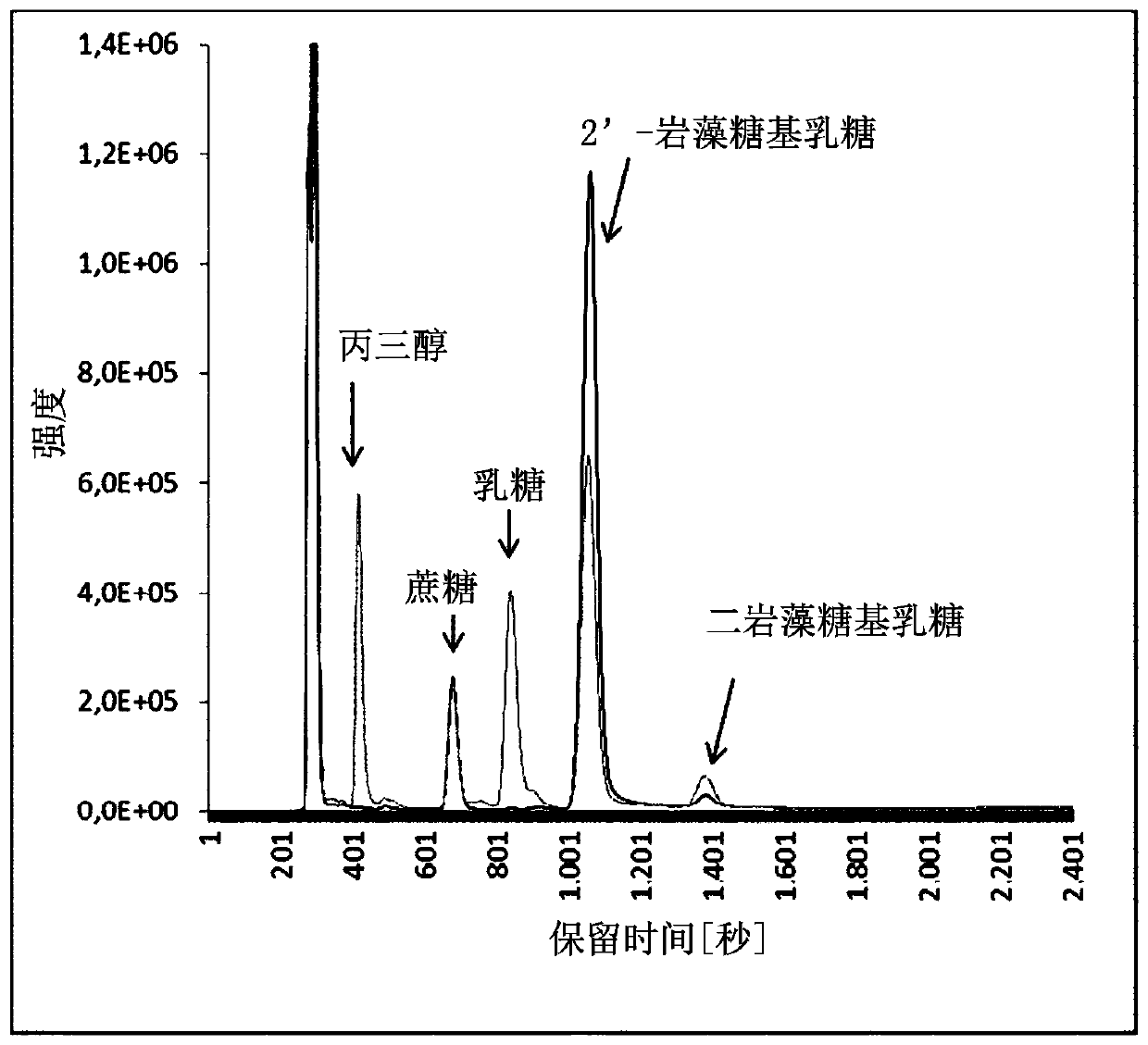
[0133] Fermentations were carried out in 3 L fermenters at 30°C and pH 7.0; the pH was adjusted by titration with 25% ammonia. The strain described in Example 2 was cultivated in the mineral salt medium described in Example 2 using glycerol as a carbon source and energy source. A fermenter with an initial volume of 1 L was inoculated with a preculture grown in the same medium. Glycerol was added continuously (60% v / v) after the 2% glycerol contained in the batch was consumed. When OD 600nm When 6 was reached, lactose was added at a concentration of 0.66 M in three portions (10 ml each) (1 hour apart). Thereafter, lactose was administered in a continuous flow to maintain a lactose concentration in the fermentor of at least 10 mM. After culturing for 86 h, the final titer of 91.3 mM (44.6 g / L) 2'-fucosyllactose was reached. By changing the temperature to 42°C and expressing the β-galactosidase gene, lactose and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com