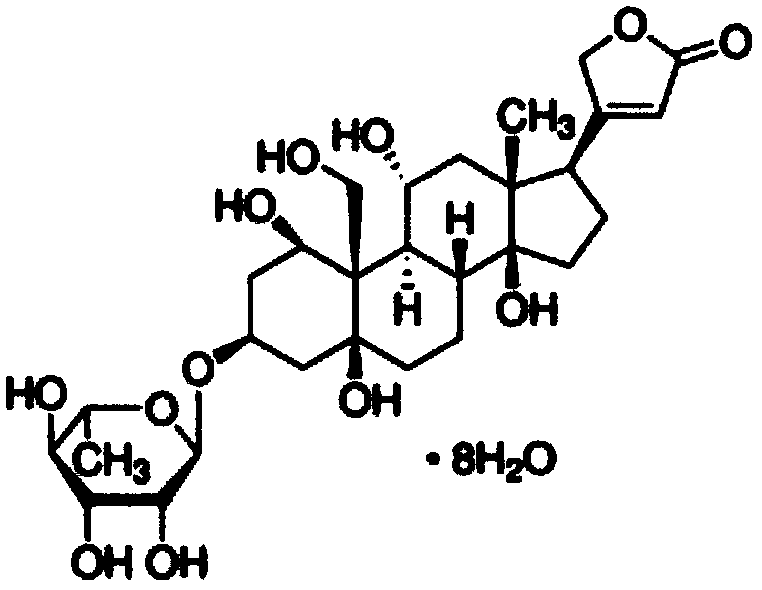
Application of ouabain in preparation of anti-non-alcoholic fatty liver drug
A non-alcoholic, fatty liver technology applied in the field of sodium potassium ATPase inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Establishment of mouse non-alcoholic fatty liver model
[0025] 1.1 Experimental materials
[0026] 6-8 week-old male C57BL / 6 mice and 6-week-old male Ob / ob mice were from Nanjing University-Institute of Model Animals; 60% high-fat diet (D12492) and isocaloric control diet (D12450J) From the American ResearchDiets company.
[0027] 1.2 Experimental method
[0028] 1.2.1 Non-alcoholic fatty liver model in mice induced by high-fat diet feeding
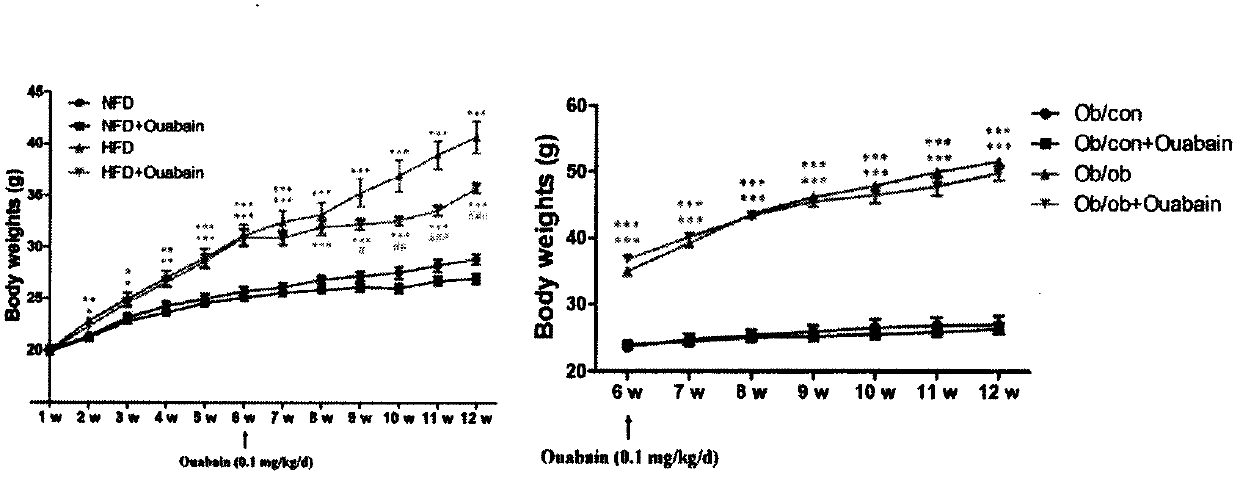
[0029] Male C57BL / 6 mice aged 6-8 weeks were adaptively fed for several days and randomly divided into low-fat diet (NFD) and high-fat diet (HFD). Mice in NFD group were fed with D12450J feed every day for 12 consecutive weeks, while HFD mice were fed with D12492 high-fat feed every day for 12 consecutive weeks. All mice were given free access to drinking water, and the body weight of the mice was monitored weekly.
[0030] 1.2.2 Non-alcoholic fatty liver model in Ob / ob transgenic mice
[0031] Ob / ob mice are homoz...
Embodiment 2
[0034] Example 2 Oouabain reduces the accumulation of fat in the liver of mice in the state of non-alcoholic fatty liver disease
[0035] 2.1 Experimental materials
[0036] Oabain was purchased from Sigma, USA; Triglyceride (TG) and Cholesterol (TC) assay kits were purchased from Nanjing Jiancheng Bioengineering Institute; HE staining kits were purchased from Wuhan Sewell Biotechnology Co., Ltd.; Oil Red O reagent Purchased from Sigma, USA.
[0037] 2.2 Experimental method
[0038] 2.2.1 Administration method and sample collection of ouabain
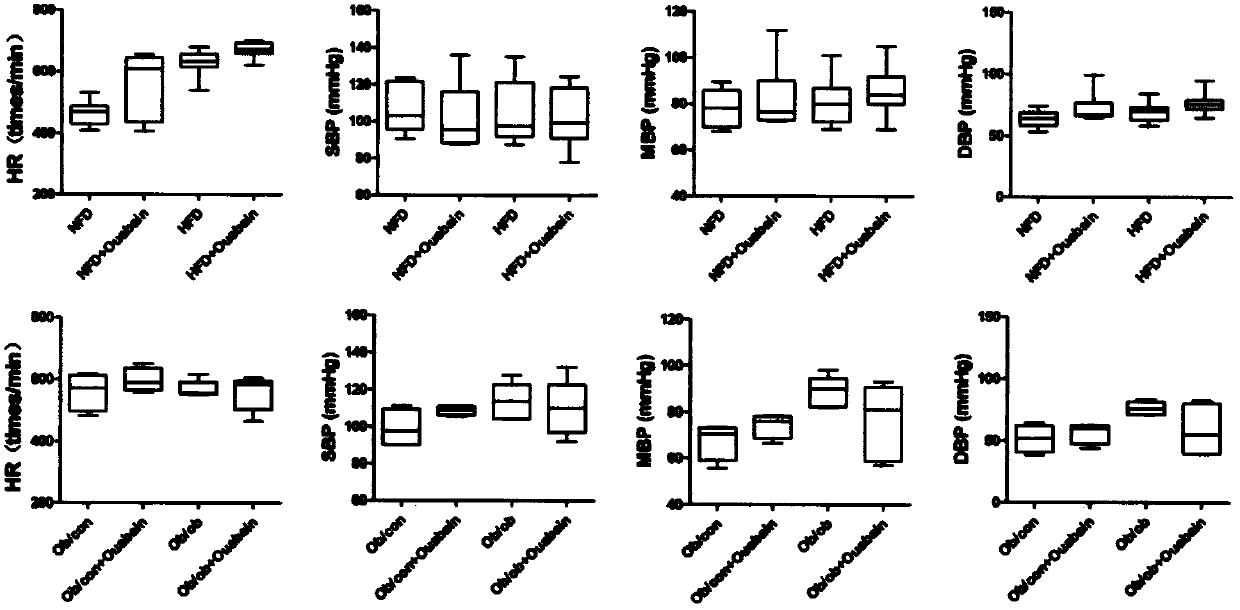
[0039] Male C57BL / 6 mice aged 6-8 weeks were randomly divided into 4 groups (8 mice in each group), and 2 groups of mice were fed with NFD and HFD diets, respectively. After 6 weeks, the two groups of mice fed with NFD diet were given intraperitoneal injection of 0.1 mg / kg ouabain (NFD+Ouabain group) or the same amount of normal saline (NFD group) respectively, and injected continuously for 6 weeks; The two groups of mice were also ...
Embodiment 3
[0053] Example 3 Oouabain reduces liver damage in mice in the state of non-alcoholic fatty liver disease
[0054] 3.1 Experimental materials
[0055] The experimental mice were the same as in Example 1; the source of ouabain was the same as in Example 2; Aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) assay kits were purchased from Nanjing Jiancheng Institute of Bioengineering.
[0056] 3.2 Experimental method
[0057] 3.2.1 Mice modeling and administration methods
[0058] The mouse model and the administration method of ouabain are the same as those in Example 1 and Example 2.
[0059] 3.2.2 Detection of AST, ALT and ALP activity in mouse serum
[0060] The serum of the mice was separated, and the activities of AST, ALT and ALP in the serum of the two mouse models were determined according to the kit instructions.
[0061] 3.3 Experimental results
[0062] The activities of AST, ALT, and ALP in the serum of high-fat-induc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com