Chimeric Antigen Receptor Expressed on the Surface of T Lymphocytes and Its Application
A chimeric antigen receptor and lymphocyte technology, applied in the field of genetically modified cells and tumor treatment, can solve the problem of not being able to target CAR-T cells at the same time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of T lymphocytes targeting MUC1 and NKG2D ligands and T lymphocytes targeting MUC1
[0028] 1. Synthesis and Amplification
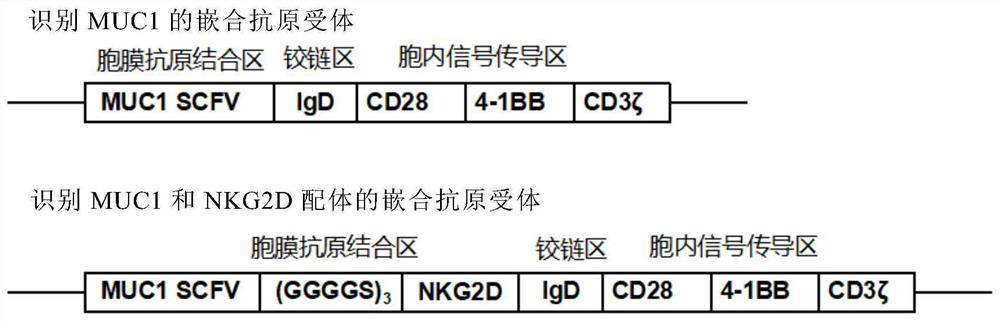
[0029] 1) Synthesize the nucleotide sequence (SEQ ID NO: 2) encoding the chimeric antigen receptor targeting MUC1 and NKG2D ligands of the present invention, and the chimeric antigen receptor 1 (SCFV-IgD–CD28– The nucleotide sequence (SEQID NO:3) of 4-1BB-CD3ζ) was synthesized by Suzhou Synbio Biotechnology Co., Ltd.;
[0030] 2) After amplifying SEQ ID NO: 2 and SEQ ID NO: 3, clone them into the lentiviral expression vector pGreen puro (purchased from Aidi Gene (Add gene) company), respectively. The structure diagram of pGreen puro is as follows Figure 5 As shown, SEQ ID NO: 2 and SEQ ID NO: 3 were connected to the vector through restriction sites BamHI and EcoRI to obtain recombinant lentiviral vector 1 and recombinant lentiviral vector 2 respectively, and the recombinant vectors were obtained after restriction restrictio...
Embodiment 2
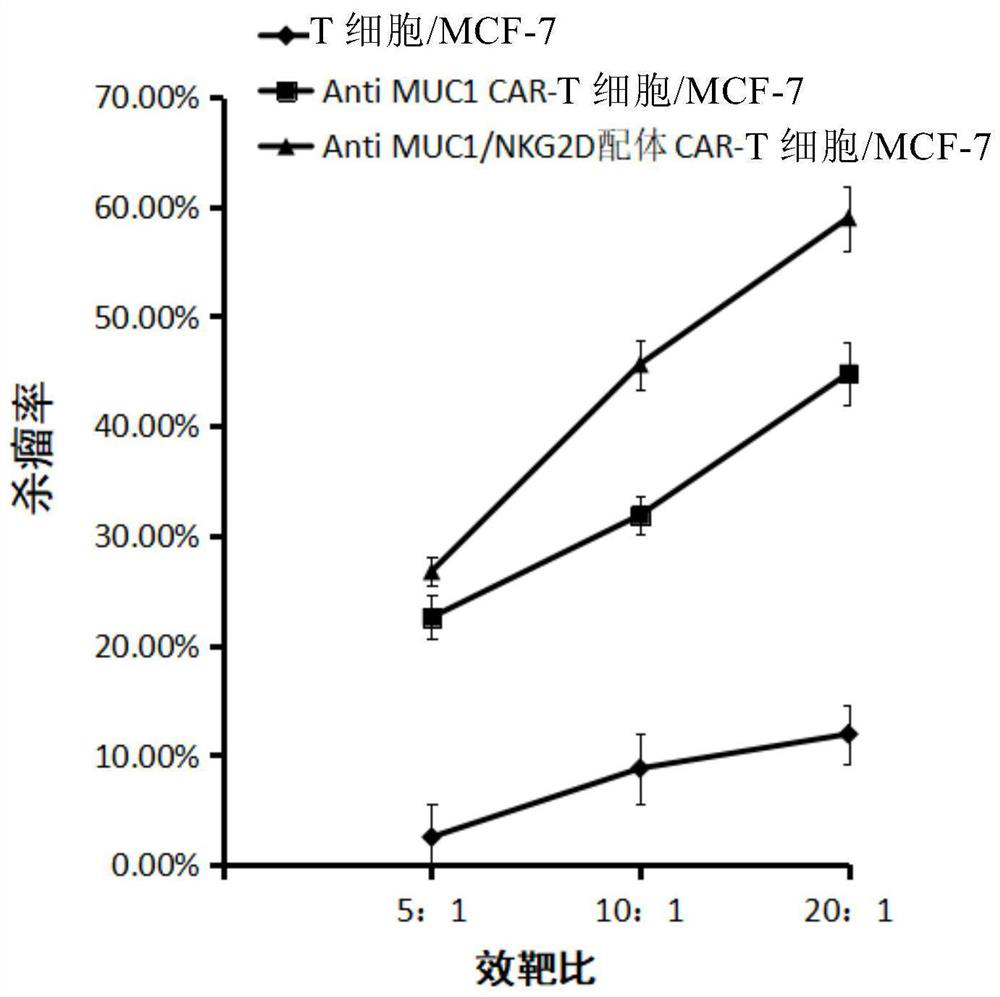
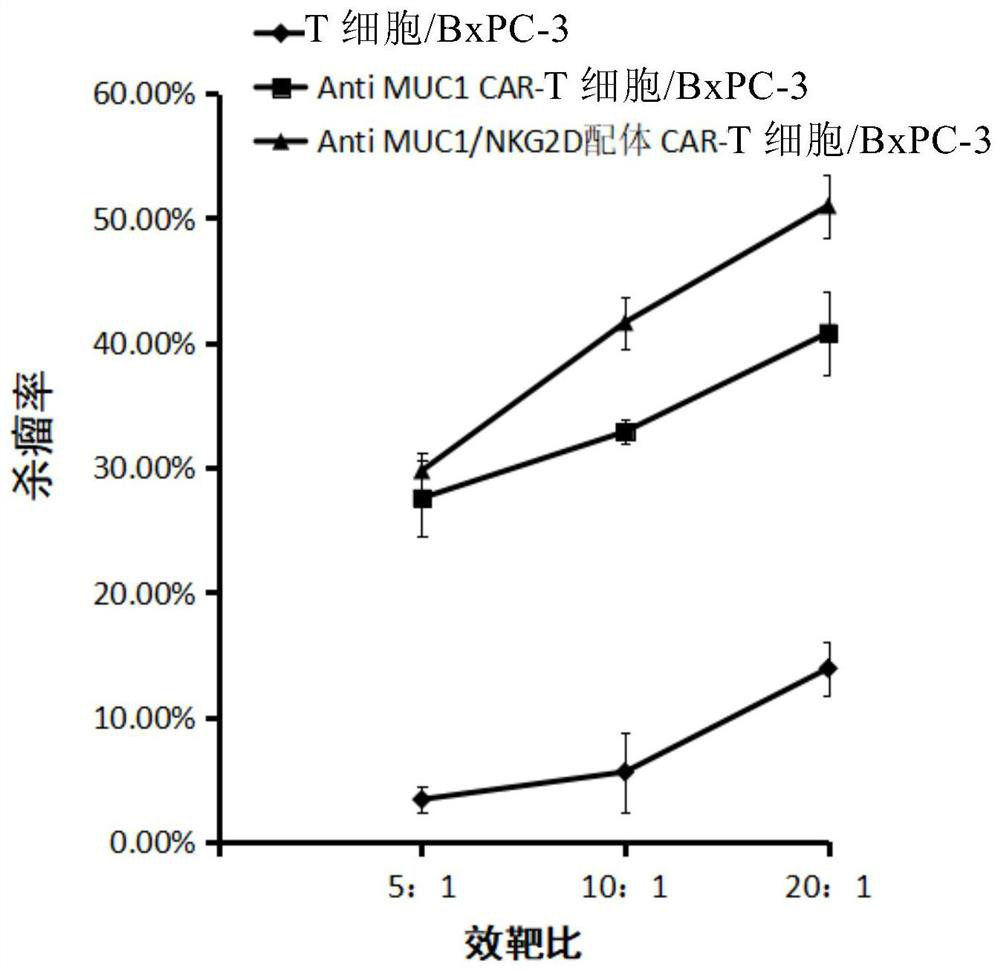
[0057] Example 2 The present invention provides verification of the effect of genetically modified CD8+ T lymphocytes in treating tumors in vitro
[0058] 1. The breast cancer cell line MCF-7 stably expressing MUC1 and NKG2D ligands was used as target cells (purchased from ATCC) and divided into 3 groups.
[0059] The CD8+ T lymphocytes targeting MUC1 and NKG2D ligands prepared in Example 1 were used as effector cells 1; the CD8+ T lymphocytes targeting MUC1 prepared in Example 1 were used as effector cells 2; there were also uninfected virus CD8+ T cells as effector cells 3;
[0060] 1) In the experimental group (Anti MUC1 / NKG2D ligand CAR-T cells / MCF-7), the effector cell 1 was added to the first group of target cells at an effect-to-target ratio of 5:1, 10:1, and 20:1;
[0061] 2) For control group 1 (Anti MUC1CAR-T cells / MCF-7), effector cells 2 were added to the second group of target cells at an effect-to-target ratio of 5:1, 10:1, and 20:1;
[0062] 3) Control group 2...
Embodiment 3
[0072] Example 3 The present invention provides verification of the effect of gene-modified T lymphocytes in treating tumors in vivo
[0073] In 20 nude mice (6 weeks old, body weight 18 ~ 20g, purchased from Guangdong Provincial Medical Experimental Animal Center) subcutaneously injected 5x 10 6 MCF-7 breast cancer cells (purchased from ATCC), observe whether tumors appear in mice, and wait for tumors to grow to 60mm 3 size, they were randomly divided into 4 groups.
[0074] 1) For the control group (control / MCF-7), 200ul of normal saline was injected into the tail vein twice a week;
[0075] 2) In the T cell therapy group (T cell / MCF-7), 1×10 CD8+ T cells without virus infection were injected into the tail vein respectively. 7 each time, 2 times a week;
[0076] 3) The T lymphocyte therapy group targeting MUC1 (Anti MUC1CAR-T cells / MCF-7), the T lymphocytes targeting MUC1 prepared in Example 1 were injected into the tail vein 1×10 7 each time, 2 times a week;
[0077]4)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com