Triarylamine organic compound and application thereof
A technology of organic compounds and triarylamines, applied in the field of triarylamine organic compounds, can solve problems such as differences, and achieve the effects of improving luminous efficiency, improving recombination efficiency, and improving exciton utilization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
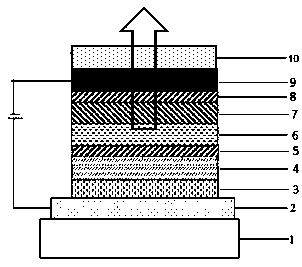
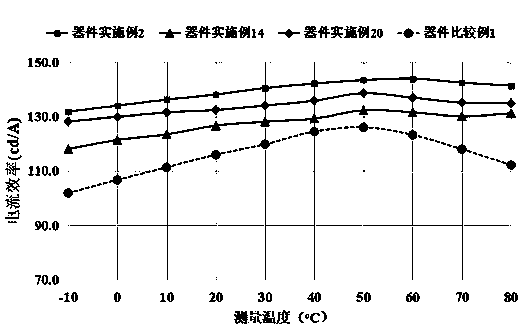
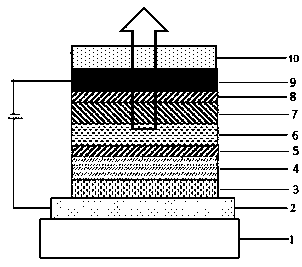
Image
Examples
Embodiment 1
[0050] Embodiment 1: the synthesis of compound 2
[0051]
[0052] (1) In a 250ml three-neck flask, under the protection of nitrogen, add 0.012mol 1,3-dibromo-5-iodobenzene, 0.01mol raw material A-1, 150ml toluene and stir to mix, then add 5×10 -5 mol Pd 2 (dba) 3 , 5×10 -5 mol tri-tert-butylphosphine, 0.03mol sodium tert-butoxide, heated to 105°C, refluxed for 24 hours, sampling plate, showed no amino compound remaining, the reaction was complete; naturally cooled to room temperature, filtered, and the filtrate was rotary evaporated to no fraction , through a neutral silica gel column to obtain intermediate M-1 with a purity of 99.7% and a yield of 91.4%.
[0053] Elemental analysis structure (molecular formula C 29 h 29 Br 2 N): Theoretical C, 63.17; H, 5.30; Br, 28.98; N, 2.54; Found: C, 63.14; H, 5.31; Br, 28.98; N, 2.56. ESI-MS (m / z) (M+): The theoretical value is 551.37, and the measured value is 550.84.
[0054] (2) In a 500ml three-neck flask, under the prot...
Embodiment 2
[0056] Embodiment 2: the synthesis of compound 18
[0057]
[0058] (1) The synthesis steps of intermediate M-2 are similar to those of intermediate M-1, except that raw material A-1 is replaced by raw material A-3;
[0059] Elemental analysis structure (molecular formula C 33 h 25 Br 2 N): Theoretical C, 66.57; H, 4.23; Br, 26.84; N, 2.35; Found: C, 66.55; H, 4.23; Br, 26.84; N, 2.37. ESI-MS (m / z) (M+): The theoretical value is 595.38, and the measured value is 594.93.
[0060] (2) The synthesis steps of compound 18 are similar to those of compound 2, except that intermediate M-1 is replaced by intermediate M-2, and raw material A-2 is replaced by raw material A-4;
[0061] Elemental analysis structure (molecular formula C 85 h 77 N 3 ): theoretical value C, 89.51; H, 6.81; N, 3.68; test value: C, 89.45; H, 6.83; N, 3.72. ESI-MS (m / z) (M+): The theoretical value is 1140.57, and the measured value is 1139.08.
Embodiment 3
[0062] Embodiment 3: the synthesis of compound 48
[0063]
[0064] (1) The synthesis steps of intermediate M-3 are similar to those of intermediate M-1, except that raw material A-1 is replaced by raw material A-5;
[0065] Elemental analysis structure (molecular formula C 38 h 41 Br 2 N): theoretical C, 67.96; H, 6.15; Br, 23.80; N, 2.09; found: C, 67.92; H, 6.16; Br, 23.80; N, 2.12. ESI-MS (m / z) (M+): The theoretical value is 671.56, and the measured value is 670.95.
[0066] (2) The synthesis steps of compound 48 are similar to those of compound 2, except that intermediate M-1 is replaced by intermediate M-3, and raw material A-2 is replaced by raw material A-6;
[0067] Elemental analysis structure (molecular formula C 78 h 93 N 3 ): theoretical value C, 87.34; H, 8.74; N, 3.92; test value: C, 87.27; H, 8.76; N, 3.97. ESI-MS (m / z) (M+): The theoretical value is 1072.62, and the measured value is 1071.47.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com