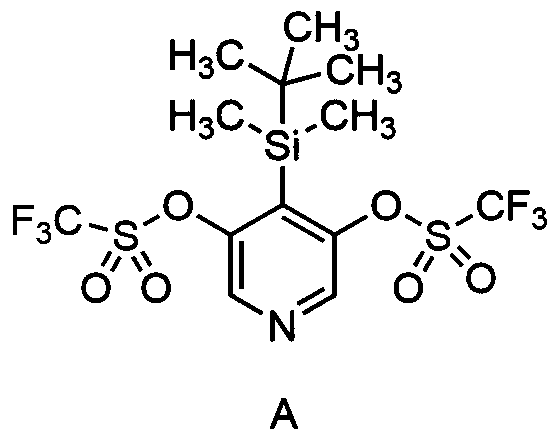
Pyridine alkyne precursor and synthesis method thereof
A synthetic method and technology of pyridyne, applied in the field of pyridyne precursor and its synthesis, to achieve good solubility, stable chemical properties, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0023] The aryne precursor of the present invention is composed of five steps:
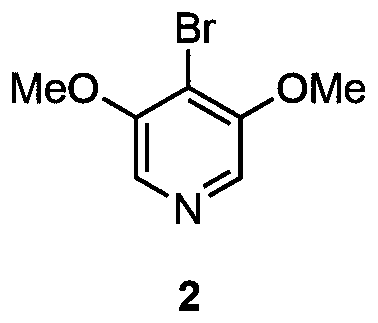
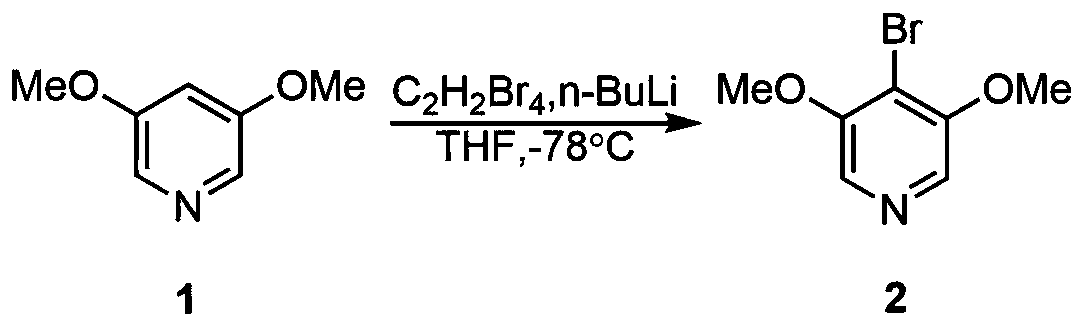
[0024] The first step, the synthesis of 4-bromo-3,5-dimethoxypyridine
[0025] The structural formula 2 of 4-bromo-3,5-dimethoxypyridine is as follows:
[0026] The synthetic reaction process of this 4-bromo-3,5-dimethoxypyridine:
[0027]
[0028] 3,5-dimethoxypyridine (1.4g, 10mmol, 1.0equiv.) of structural formula 1 was dissolved in tetrahydrofuran
[0029]
[0030] (30mL), cooled to -78°C, added n-butyllithium (n-BuLi) (2.5M n-hexane solution, 4.8mL, 12mmol, 1.2equiv.), stirred for five minutes, added 1,1,2,2 -Tetrabromoethane (1.75mL, 15mmol, 1.5equiv.), returned to room temperature. Post-treatment and purification by silica gel column chromatography yielded 1.14 g of 4-bromo-3,5-dimethoxypyridine 2 with a yield of 52%.
[0031] The second step, the synthesis of 4-bromo-3,5-dihydroxypyridine
[0032] The structural formula 3 of 4-bromo-3,5-dihydroxypyridine is as follows:
[0033]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com