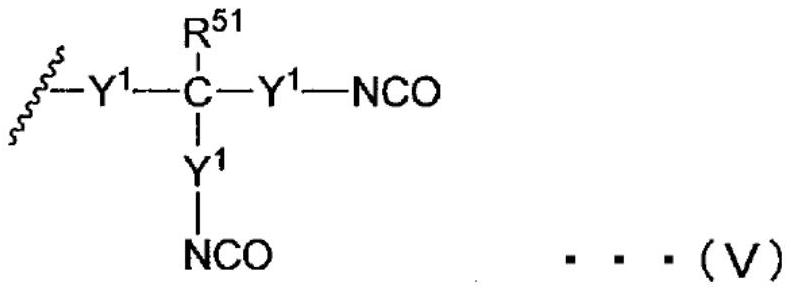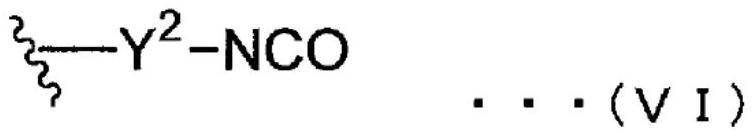Polyisocyanate composition, blocked polyisocyanate composition, hydrophilic polyisocyanate composition, coating composition and coating film
A technology of polyisocyanate and triisocyanate, applied in polyurea/polyurethane coatings, coatings, etc., can solve the problems of polyisocyanate viscosity increase, difficult handling, poor drying performance, etc., and achieve excellent water resistance, reduced solvent content, and good drying performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 Embodiment approach
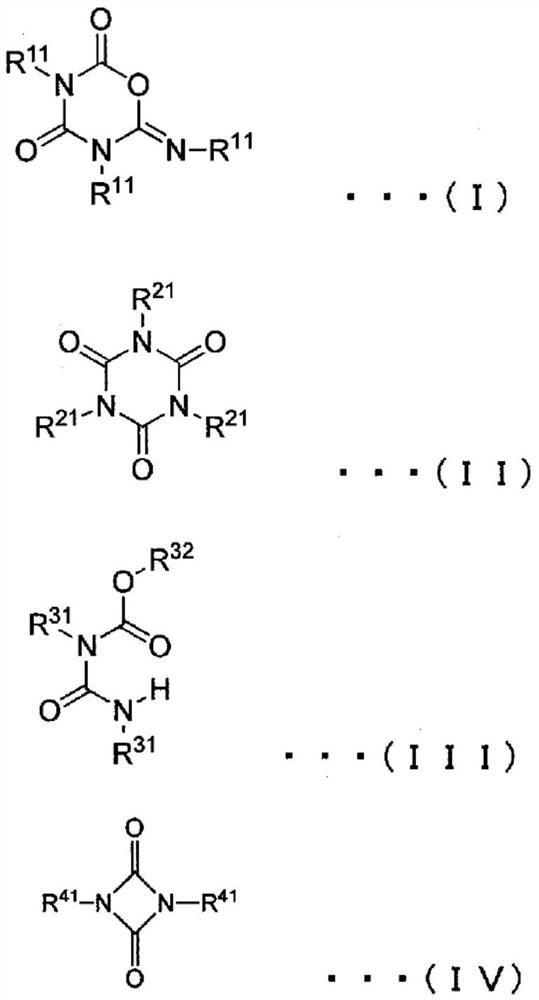
[0150] The polyisocyanate composition of 1st Embodiment of this invention contains the polyisocyanate compound represented by General formula (I), (II), (III), or (IV).
[0151]
[0152] [in general formula (I), (II), (III) and (IV), there are multiple R 11 , R 21 , R 31 and R 41 Each is independently an organic group, and there are multiple R 11 , R 21 , R 31 and R 41 At least one of them is a group represented by general formula (V) or (VI). There are multiple R 11 , R 21 , R 31 and R 41 are optionally the same or different from each other. In general formula (III), R 32 It is a residue obtained by removing one hydroxyl group from a monohydric or higher alcohol. ]
[0153]
[0154] [in the general formula (V), there are a plurality of Y 1 They are each independently a single bond, or a divalent hydrocarbon group having 1 to 20 carbon atoms that optionally includes an ester structure and / or an ether structure. There are multiple Y's 1 are optionally the...
Embodiment
[0726] Hereinafter, specific examples and comparative examples are given to describe embodiments of the present invention more specifically, but the embodiments of the present invention are not limited to the following examples and comparative examples unless the gist is exceeded.
[0727] The physical properties of the polyisocyanate compositions in Examples (1-1)-1 to (1-1)-28 and Comparative Examples (1-1)-1 to (1-1)-10 were measured as follows. In addition, "parts" and "%" mean "parts by mass" and "% by mass" unless otherwise specified.
[0728]
[0729]The viscosity was measured at 25° C. using an E-type viscometer (manufactured by TOKIMEC Corporation). For the measurement, a standard rotor (1°34'×R24) was used. The rotation speed is as follows.
[0730] 100rpm (less than 128mPa·s)
[0731] 50rpm (128mPa·s~256mPa·s)
[0732] 20rpm (256mPa·s~640mPa·s)
[0733] 10rpm (640mPa·s~1280mPa·s)
[0734] 5rpm (1280mPa·s~2560mPa·s)
[0735]
[0736] The NCO content (mass ...
Synthetic example (1-1
[0799] Synthesis of NTI
[0800] Dissolve 1060 g of 4-aminomethyl-1,8-octamethylenediamine (hereinafter sometimes referred to as "triamine") in 1500 g of methanol in a four-necked flask equipped with a stirrer, a thermometer, and a gas inlet tube. While cooling, 1800 ml of 35% concentrated hydrochloric acid was slowly added dropwise thereto. Methanol and water were removed under reduced pressure, concentrated, and dried at 60° C. / 5 mmHg for 24 hours to obtain triamine hydrochloride as a white solid. The obtained triamine hydrochloride 650g was suspended in 5000g of o-dichlorobenzene in the form of micropowder, the reaction solution was heated up while stirring, and phosgene was blown into at a speed of 200g / hour when it reached 100°C, and then Continue to raise the temperature and keep it at 180° C., and continue blowing in phosgene for 12 hours. After distilling off dissolved phosgene and solvent under reduced pressure, carry out vacuum distillation to obtain colorless and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com