Biomimetic nanoparticles for gas production and anti-tumor activity catalyzed by bioenzymes and preparation method thereof
A biomimetic nano-anti-tumor technology, applied in anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., to achieve good biocompatibility, reduce toxic and side effects, and improve curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (1) Dissolve 5 mg of glucose oxidase in 0.5 ml of water to prepare a glucose oxidase solution with a concentration of 10 mg / mL; dissolve 2 mg of manganese carbonyl compound and 100 mg of polylactic acid-glycolic acid copolymer in 5 mL of organic solution to prepare Manganese carbonyl compound (0.4mg / mL)-polylactic acid-glycolic acid (20mg / mL) solution; pre-cool all solutions in an environment of 1-5°C, and set aside.
[0051] (2) Take 500 μL of glucose oxidase solution and add it to the manganese carbonyl compound-polylactic acid-glycolic acid solution in step (1), perform ultrasonic emulsification for 30 s, and the ultrasonic emulsification power is 300 W, and prepare W / O colostrum for later use.
[0052] (3) Immediately transfer the colostrum obtained in step (2) into 5 mL of 2% polyvinyl alcohol (PVA) solution, and perform ultrasonic emulsification for 2 minutes with a ultrasonic emulsification power of 200 W to prepare a W / O / W double emulsion. spare.
[0053] (4) A...
Embodiment 2
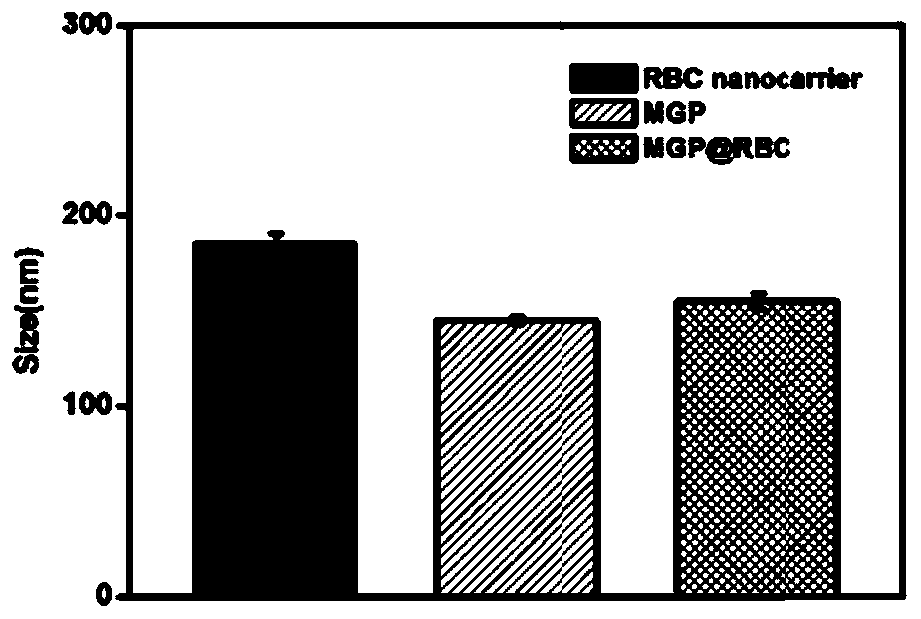
[0057] PLGA nanocarriers (MGP) and red blood cell membrane-wrapped PLGA nanocarriers (MGP@RBC) were prepared according to the method in Example 1, and PLGA nanocarriers (MGP), red blood cell membrane-wrapped PLGA nanocarriers (MGP@RBC) and red blood Membrane (RBC) was made into a solution with a concentration of 500μg / mL with deionized water, and then its particle size was measured at 37°C.
[0058] The particle size results of the two nanocarriers and the red blood cell membrane are as follows figure 1 As shown, the particle size of red blood cell membrane (RBC) is 180±4nm; the particle size of PLGA nanocarrier (MGP) is 145±2nm; the particle size of red blood cell membrane-wrapped PLGA nanocarrier (MGP@RBC) is 155.0±4nm. The results showed that the particle size of PLGA nanocarriers wrapped in red blood cell membrane (MGP@RBC) became larger, which proved the successful wrapping of red blood cell membrane.
Embodiment 3
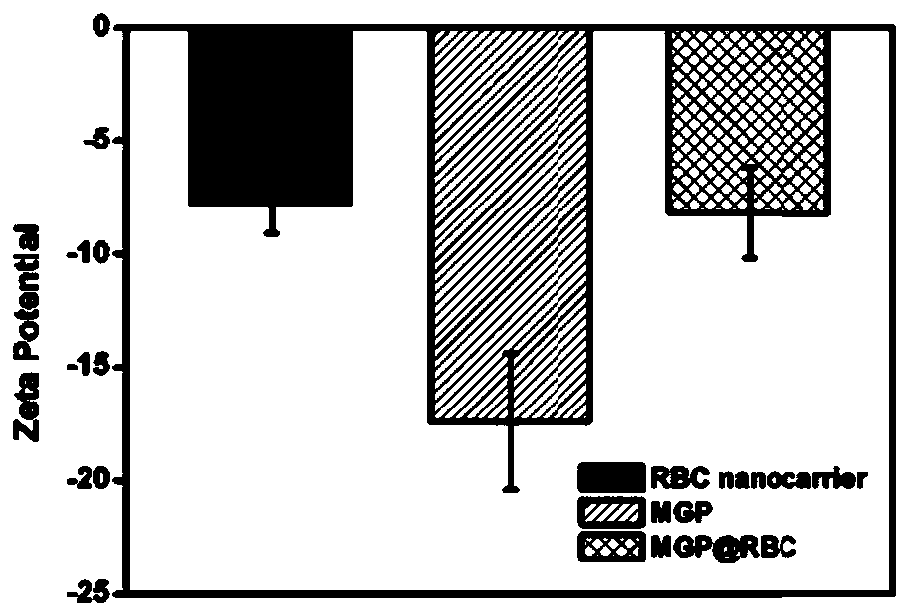
[0060] PLGA nanocarriers (MGP) and red blood cell membrane-wrapped PLGA nanocarriers (MGP@RBC) were prepared according to the method in Example 1, and PLGA nanocarriers (MGP), red blood cell membrane-wrapped PLGA nanocarriers (MGP@RBC) and red blood cell Membrane (RBC) was made into a solution with a concentration of 500 μg / mL with deionized water, and then its potential was measured at 37 °C.
[0061] The results of two nanocarriers and erythrocyte membrane potential are as follows figure 2 As shown, the potential of red blood cell membrane (RBC) is -7.8±2mV; the potential of PLGA nanocarrier (MGP) is -17.4±2mV; the potential of red blood cell membrane-wrapped PLGA nanocarrier (MGP@RBC) is -8.2±2nm. The results showed that the potential of the erythrocyte membrane-wrapped PLGA nanocarrier (MGP@RBC) presented the potential of the red blood cell membrane (RBC), which proved the successful wrapping of the red blood cell membrane.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com