Method for the detection of apolipoprotein e4
An apolipoprotein and binding agent technology, which can be used in measurement devices, instruments, disease diagnosis, etc., and can solve problems such as inability to measure signals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0251] The production of antibodies and the determination of their affinity constants
[0252] -Peptides / conjugates used for immunization:
[0253] A specific ApoE4 peptide and ApoE2 / 3 specific control peptide for immunization were synthesized, see Table 1 (JPT Technologies, Berlin, Germany). The ApoE4 peptide was synthesized with an additional N-terminal cysteine residue for coupling the peptide to bovine serum albumin (BSA). The peptide was covalently linked to BSA using Sulfolink coupling gel (Perbio-science, Bonn, Germany). The coupling procedure was performed according to Perbio's manual.
[0254] Table 1:
[0255] Peptides
ApoE area
description
Affinity constant [M -1 ]
Cys-EDVRGRLVQYR
109-119 ApoE4
2.1x 109
EDVCGRLVQYR
109-119 ApoE2 / 3
Control peptide
-
[0256] -Immunization, immune cell fusion and screening of mice:
[0257] Balb / c mice were treated with 100 μg peptide-BSA conjugate (emulsified in 100 μl Freund's complete adjuvant) on days 0 and 14 a...
Embodiment 2
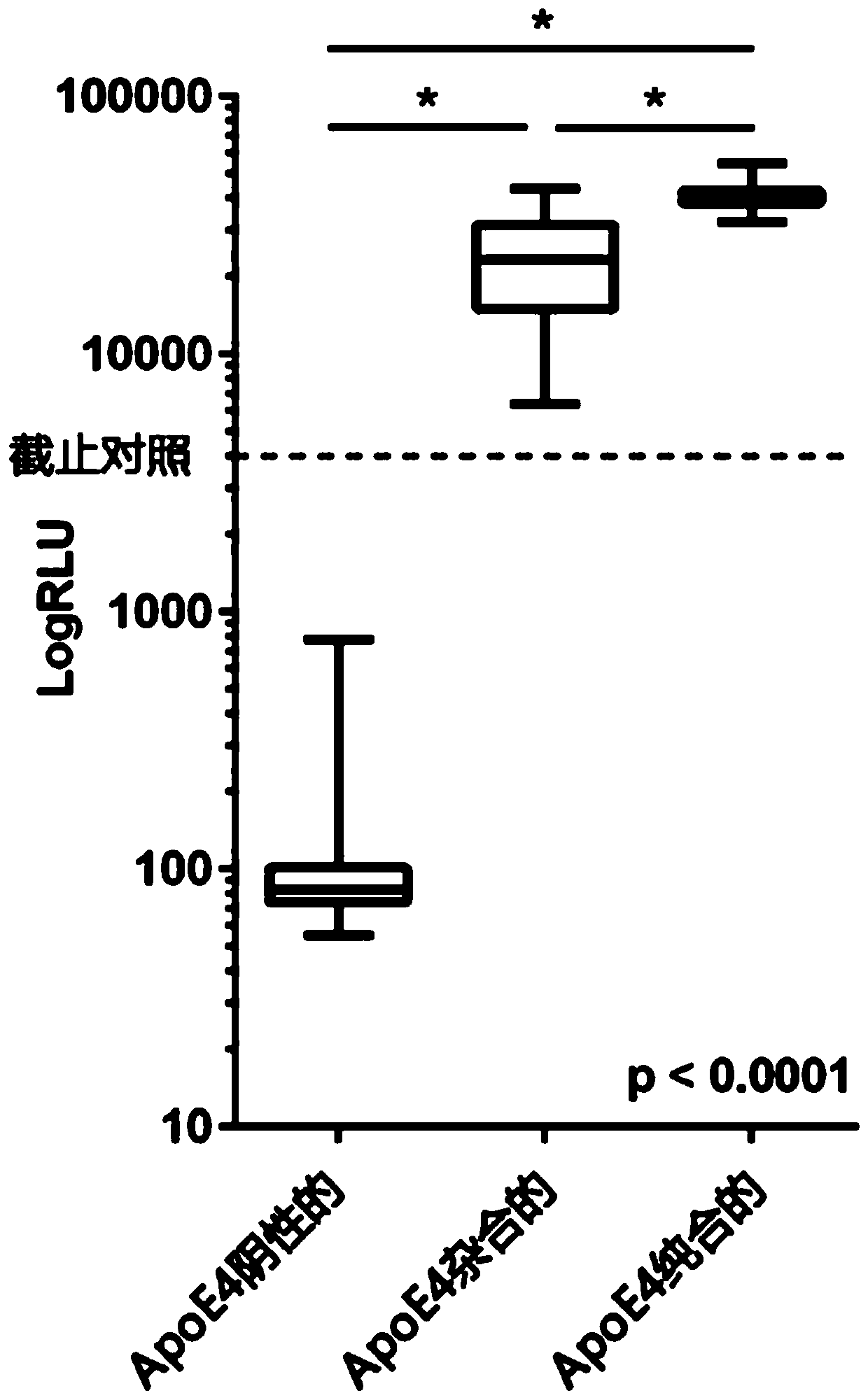
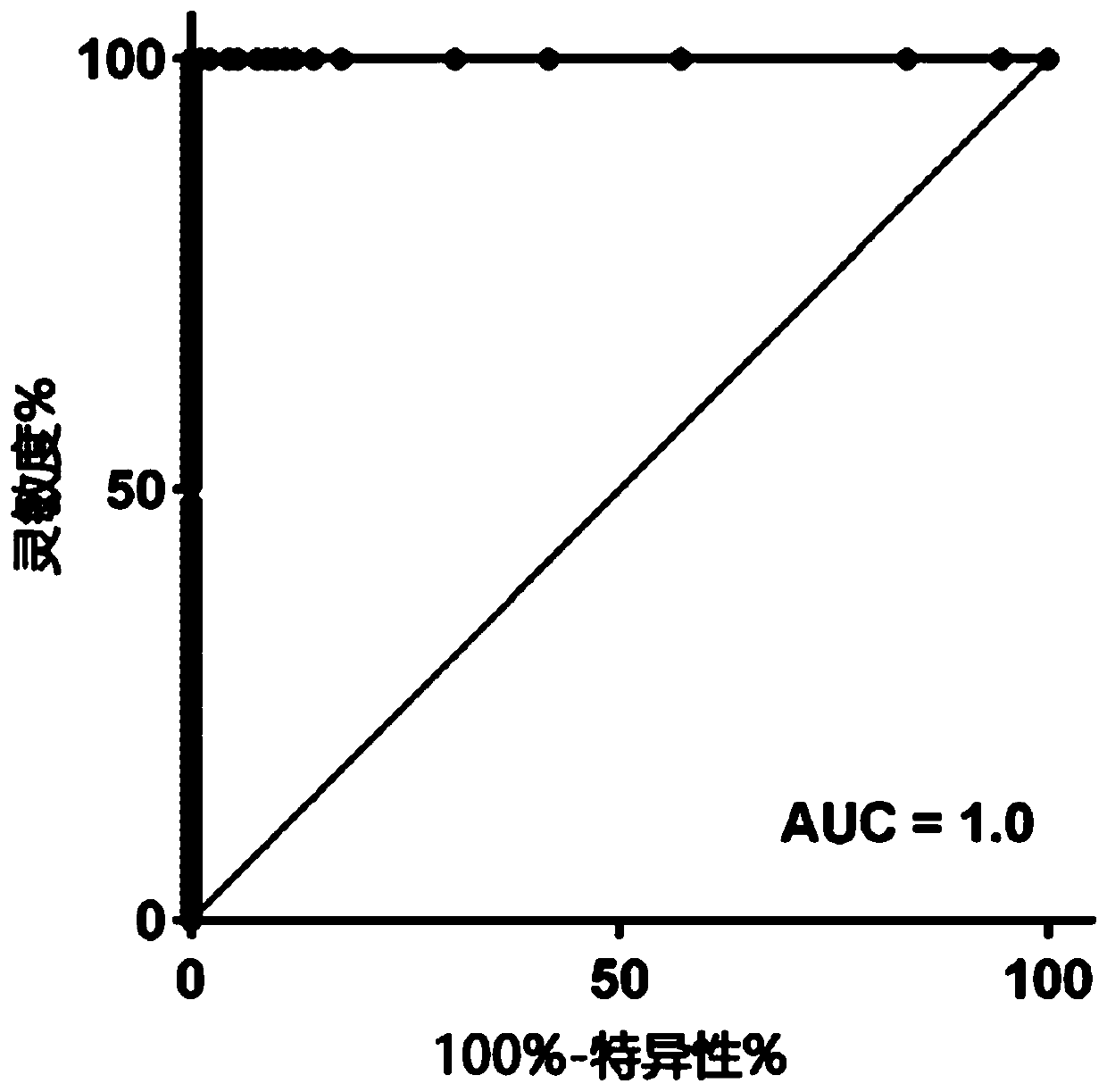
[0267] Measurement of plasma samples with known genotypes. Using plasma and whole blood matching, one can genetically analyze the ApoE status through genotyping, and use the ApoE4 assay of the present invention to measure the ApoE4 level in the matched plasma.
[0268] 2.1 Method
[0269] -Genotyping
[0270] The genetic analysis from 180 whole blood samples was performed by Medigenomix (Ebersberg, Deutschland). Isolate DNA from the sample and use Sanger sequencing technology ( Sanger&Coulson 1975 ) Sequencing the ApoE gene.
[0271] -ApoE4 assay procedure
[0272] The ApoE4 single antibody assay is performed in a one-step procedure. The plasma samples were pipetted directly into the wells of a 96-well microtiter plate (Greiner, Lumitrac 600 high binding polystyrene; 20 μl sample per well). Add ApoE4 cut-off lower limit control (recombinant ApoE4 [4μg / ml] in ApoE4-negative human plasma, 20μl per well, in duplicate) to each microplate. Add 200μl of reaction buffer (PBS, containing ...
Embodiment 3
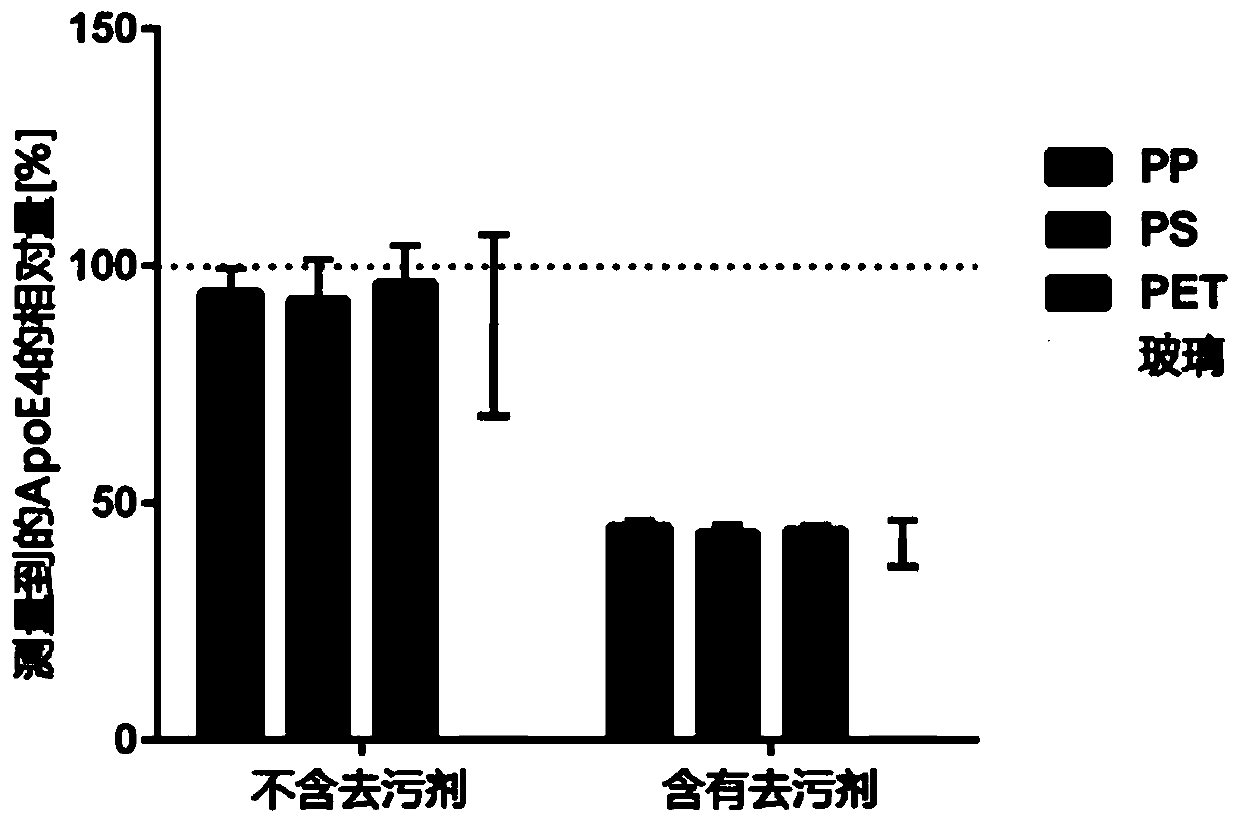
[0276] Analysis of the binding of ApoE4 to different polymers and glass surfaces with and without detergents.
[0277] 3.1 Method
[0278] -Pre-warming bath of plasma sample on polymer or glass surface:
[0279] Divide each ApoE4 positive plasma sample into 120μl aliquots and place them in tubes of different materials (polystyrene (PS), polypropylene (PP), polyethylene terephthalate (PET) and glass) Medium temperature bath, two tubes of each material. Add 0.1% Triton X-100 to half of the aliquot before the warm bath, and treat the other half with an equal volume of PBS. Warm all tubes overnight at room temperature.
[0280] -ApoE4 single antibody assay
[0281] Pipette 20 μl of each preparation and the unpreheated control into the wells of Greiner high binding microtiter plate. The ApoE4 assay was then performed as described in Example 2. The measured ApoE4 concentration is calculated relative to the concentration of the corresponding control sample (defined as 100%).
[0282] 3.2 R...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com