Method for enriching phosphorylation peptide fragments and proteins and application
A technology for phosphorylating peptides and proteins, applied in chemical instruments and methods, peptide preparation methods, peptides, etc., can solve problems such as instability and inability to achieve specific enrichment, and achieve faster and more adequate reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
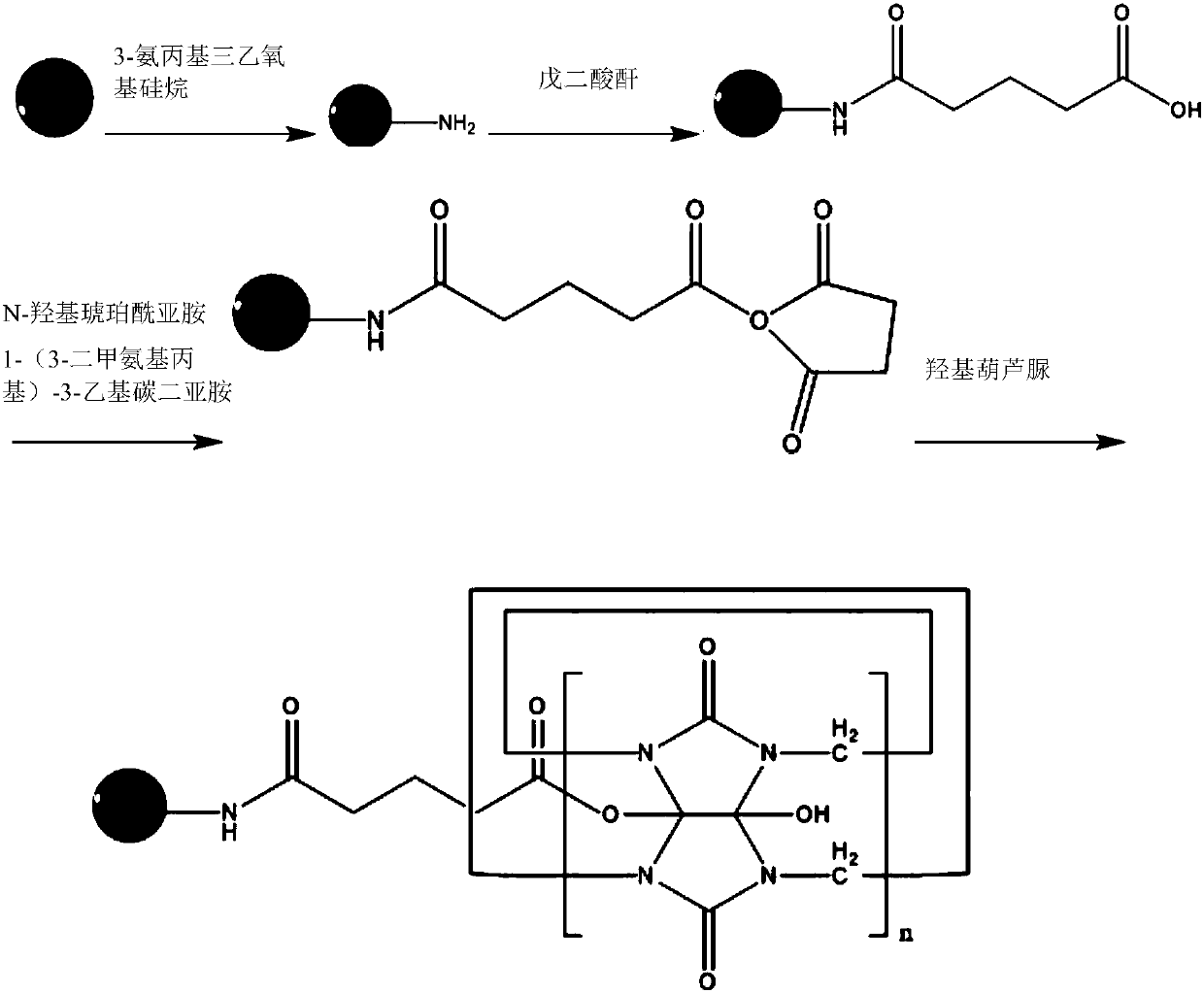
[0050] 1. Carboxylation of amantadine, the steps are as follows:
[0051] 1) Carboxylation reaction: Suspend 1 mol of 1-adamantanamine, referred to as AD1, 1 mol of 3-bromopropionic acid and 3 mol of potassium carbonate in 20 mL of acetonitrile, and reflux the mixture for 12 h.
[0052] 2) Post-treatment: the reaction mixture was filtered through diatomaceous earth, and fully washed with acetonitrile. The filtrate was concentrated, and the residue was purified through a chromatographic column using dichloromethane and methanol as mobile phases, and evaporated to dryness to obtain a colorless solid, referred to as AD1-APPA.
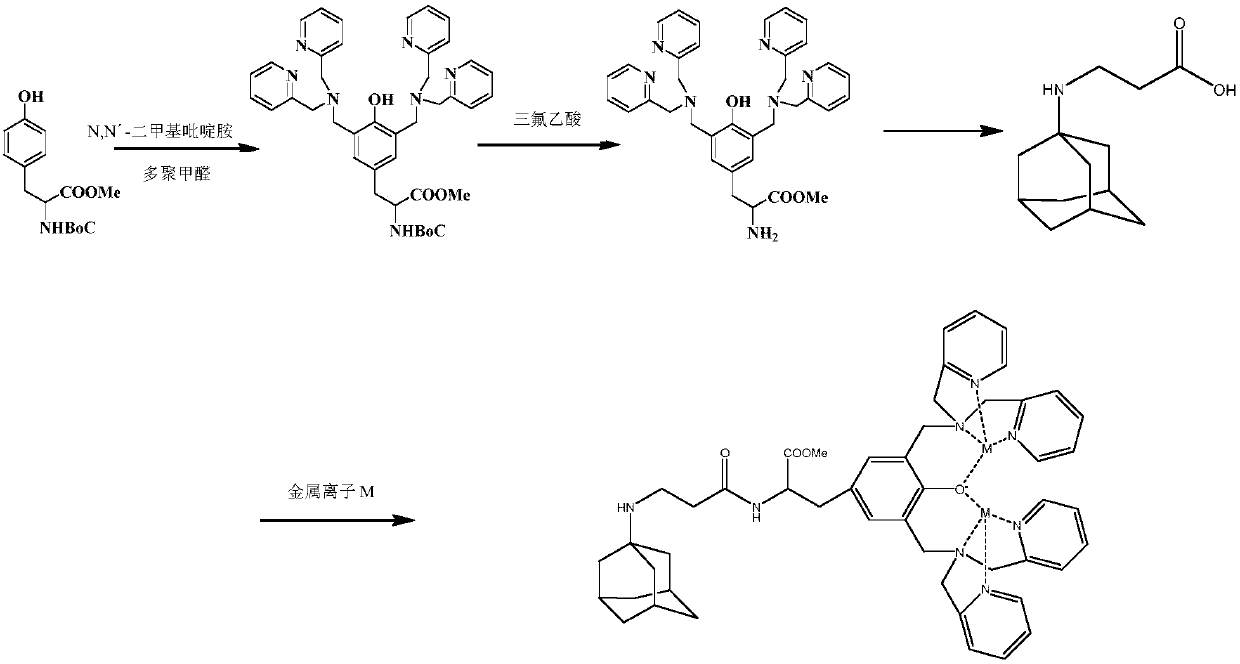
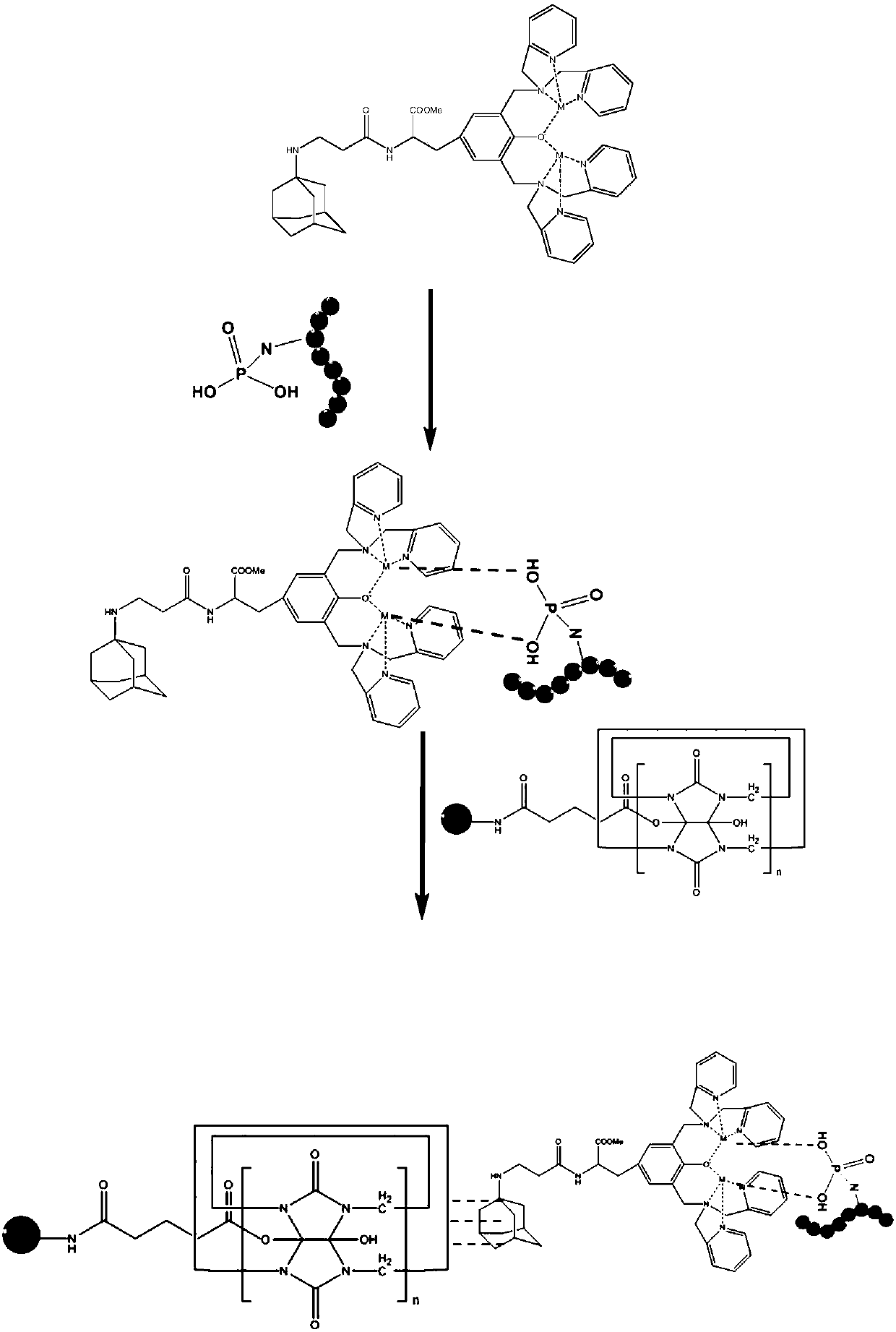
[0053] 2. A phosphoric acid recognition functional molecule: a protected precursor molecule is generated by reacting N-tert-butoxycarbonyl-L-tyrosine and N,N'-lutidine amine in the presence of paraformaldehyde, referred to as Boc-dpa, after deprotection of Boc-dpa, a functional molecule is obtained, which is called dpa for short. Proceed as follows:
[...
Embodiment 2
[0081] 1. Carboxylation of amantadine, the steps are as follows:
[0082]1) Carboxylation reaction: Suspend 500 mg of 1-adamantanamine, referred to as AD1, 510 mg of 3-bromopropionic acid and 1.37 g of potassium carbonate in 20 mL of acetonitrile, and reflux the mixture for 10 h.
[0083] 2) Post-treatment: the reaction mixture was filtered through diatomaceous earth, and fully washed with acetonitrile. The filtrate was concentrated, and the residue was purified through a chromatographic column using dichloromethane and methanol as mobile phases, and evaporated to dryness to obtain a colorless solid, referred to as AD1-APPA.
[0084] 2. A phosphoric acid recognition functional molecule: a protected precursor molecule is generated by reacting N-tert-butoxycarbonyl-L-tyrosine and N,N'-lutidine amine in the presence of paraformaldehyde, referred to as Boc-dpa, after deprotection of Boc-dpa, a functional molecule is obtained, which is called dpa for short. Proceed as follows:
...
Embodiment 3
[0112] 1. Carboxylation of amantadine, the steps are as follows:
[0113] 1) Carboxylation reaction: Suspend 400mg of 1-adamantanamine, referred to as AD1, 600mg of 3-bromopropionic acid and 1.5g of potassium carbonate in 20mL of acetonitrile, and reflux the mixture for 8h.
[0114] 2) Post-treatment: the reaction mixture was filtered through diatomaceous earth, and fully washed with acetonitrile. The filtrate was concentrated, and the residue was purified through a chromatographic column using dichloromethane and methanol as mobile phases, and evaporated to dryness to obtain a colorless solid, referred to as AD1-APPA.
[0115] 2. A phosphoric acid recognition functional molecule: a protected precursor molecule is generated by reacting N-tert-butoxycarbonyl-L-tyrosine and N,N'-lutidine amine in the presence of paraformaldehyde, referred to as Boc-dpa, after deprotection of Boc-dpa, a functional molecule is obtained, which is called dpa for short. Proceed as follows:
[0116...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com