Deoxycholic acid modified nano compound and preparation method and application thereof
A technology of nanocomposite and deoxycholic acid is applied in the field of loading insoluble active ingredients of traditional Chinese medicine, can solve problems such as poor affinity of nanocomposite, and achieve the effects of improving oral absorption, realizing oral delivery, and simple and easy preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
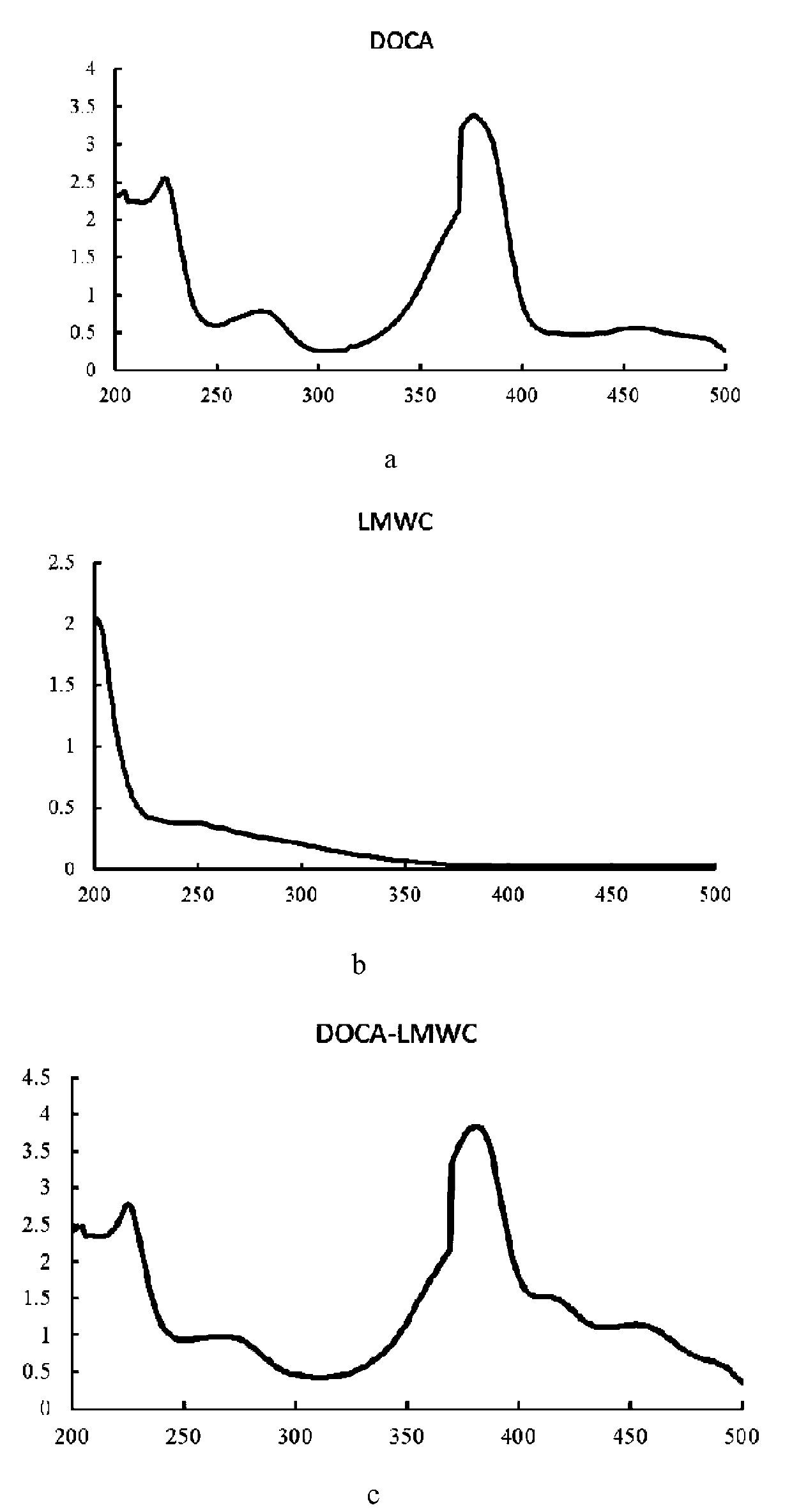
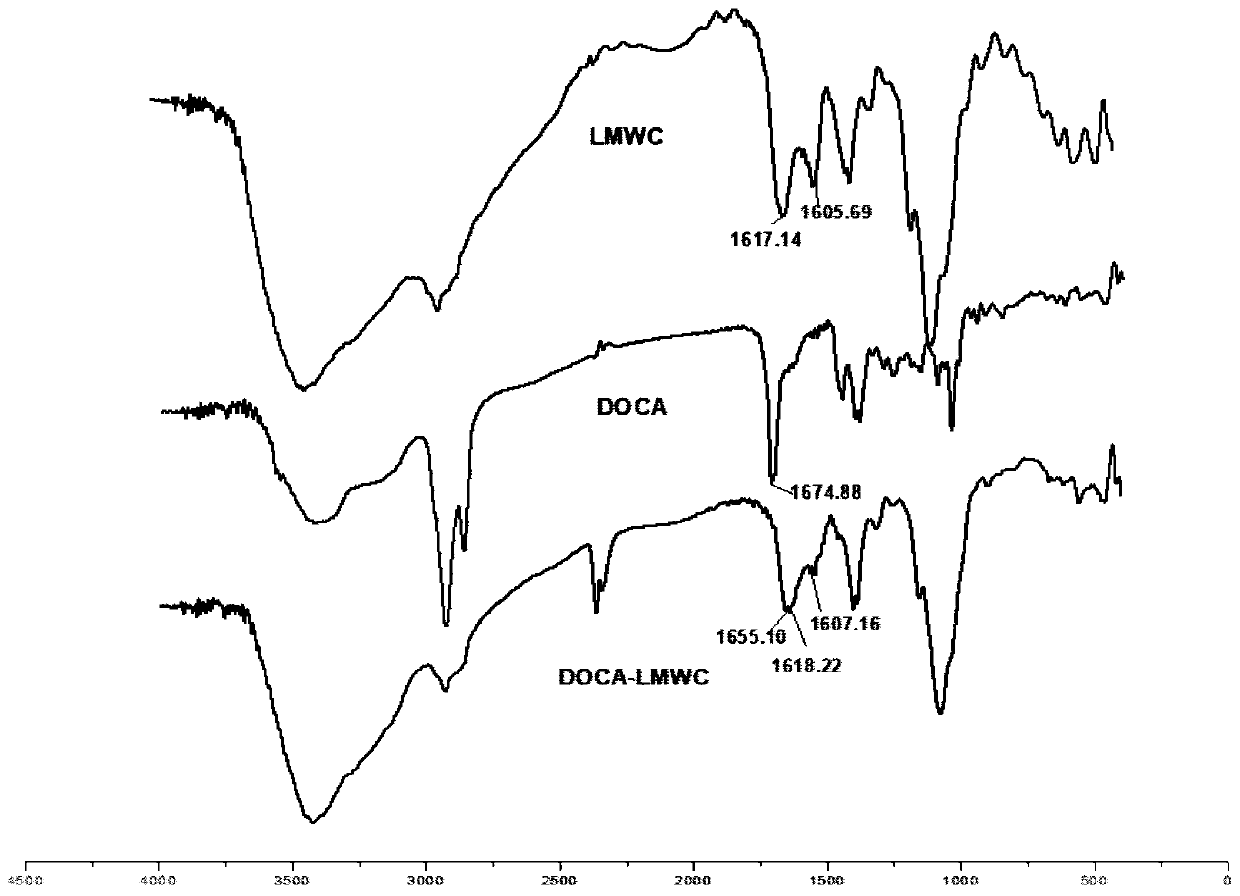
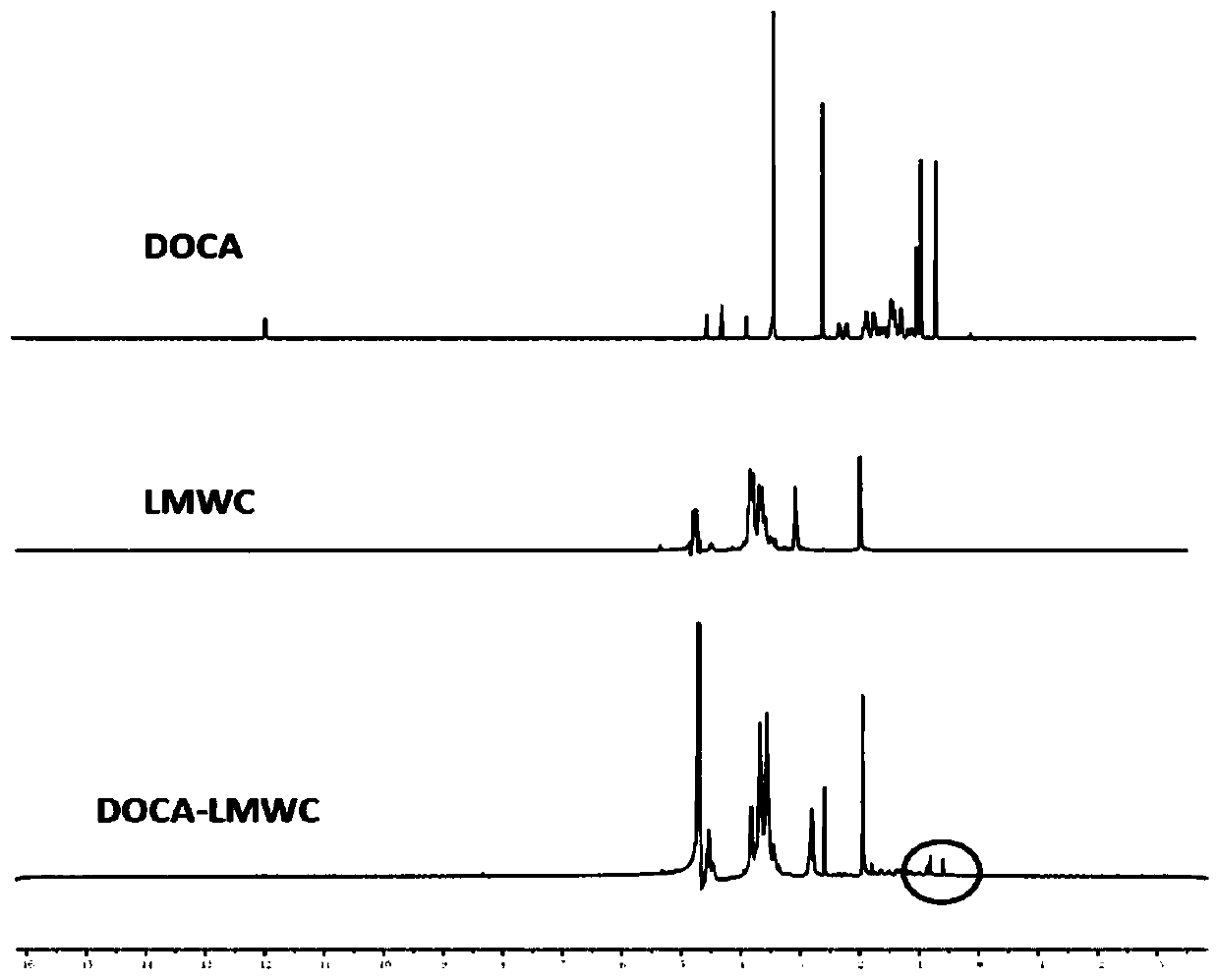
[0030] 1. Synthesis and characterization of DOCA-LMWC conjugates
[0031] (1) Weigh 31mg (0.078mmol) of deoxycholic acid (DOCA) and dissolve it in 2mL of dimethyl sulfoxide (DMSO) to make a DOCA solution. Dissolve 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) 30mg (0.156mmol) and N-hydroxysuccinimide (NHS) 18mg (0.156mmol) respectively Make an EDC / NHS solution in 1 mL DMSO. Mix all the EDC / NHS solution and DOCA solution, and react at room temperature (25-30° C.) for 2 hours to obtain activated DOCA.
[0032] (2) 60 mg of low-molecular-weight chitosan (LMWC) was dissolved in 5 mL of 90% DMSO aqueous solution to prepare LMWC solution. The activated DOCA (0.078mmol as carboxyl group) obtained in step (1) was slowly dropped into the LMWC solution (0.312mmol as amino group) under stirring, reacted at room temperature for 24h, and dialyzed with deionized water (molecular weight cut-off 3500Da) for 3d, The retentate was taken and freeze-dried at -50°C for 48 ho...
Embodiment 2
[0079] The preparation of DOCA-LMWC conjugates is the same as in Example 1, and the preparation of deoxycholic acid-modified nanocomposites is as follows: Weigh 6 mg of DOCA-LMWC conjugates and dissolve them in 2 mL of 0.2% CH 3 DOCA-LMWC acetic acid solution was made in COOH aqueous solution; 12mg CMCs was dissolved in 2ml deionized water to make CMCs solution. Add the CMCs solution dropwise to the DOCA-LMWC acetic acid solution under stirring, react at 25°C for 30 minutes until the solution appears blue opalescence, stop the reaction, and obtain the deoxycholic acid-modified nanocomposite (DOCA-LMWC / CMCs NCs) About 4ml. The particle size of the obtained nanocomposite is 230.4±1.5nm, the PDI is 0.29±0.01, and the Zeta: is 32.5±0.5mV. The preparation of nanocomposite-wrapped rhein is the same as in Example 1.
Embodiment 3
[0081] The preparation of DOCA-LMWC conjugates is the same as in Example 1, and the preparation of deoxycholic acid-modified nanocomposites is as follows: Weigh 12 mg of DOCA-LMWC conjugates and dissolve them in 2 mL of 0.2% CH 3DOCA-LMWC acetic acid solution was made in COOH aqueous solution; 6mg CMCs was dissolved in 2ml deionized water to make CMCs solution. Add the CMCs solution dropwise to the DOCA-LMWC acetic acid solution under stirring, react at 25°C for 30 minutes until the solution appears blue opalescence, stop the reaction, and obtain the deoxycholic acid-modified nanocomposite (DOCA-LMWC / CMCs NCs) About 4ml. The particle size of the obtained nanocomposite is 166.3±1.0nm, the PDI is 0.15±0.02, and the Zeta: is 42.3±0.7mV. The preparation of nanocomposite-wrapped rhein is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com