Application of styryl cyclohexene malononitrile derivative in preparation of anti-lung cancer drugs
A technology of styrene and malononitrile, applied in the field of application of styryl cyclohexallyl malononitrile derivatives in the preparation of anti-lung cancer drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
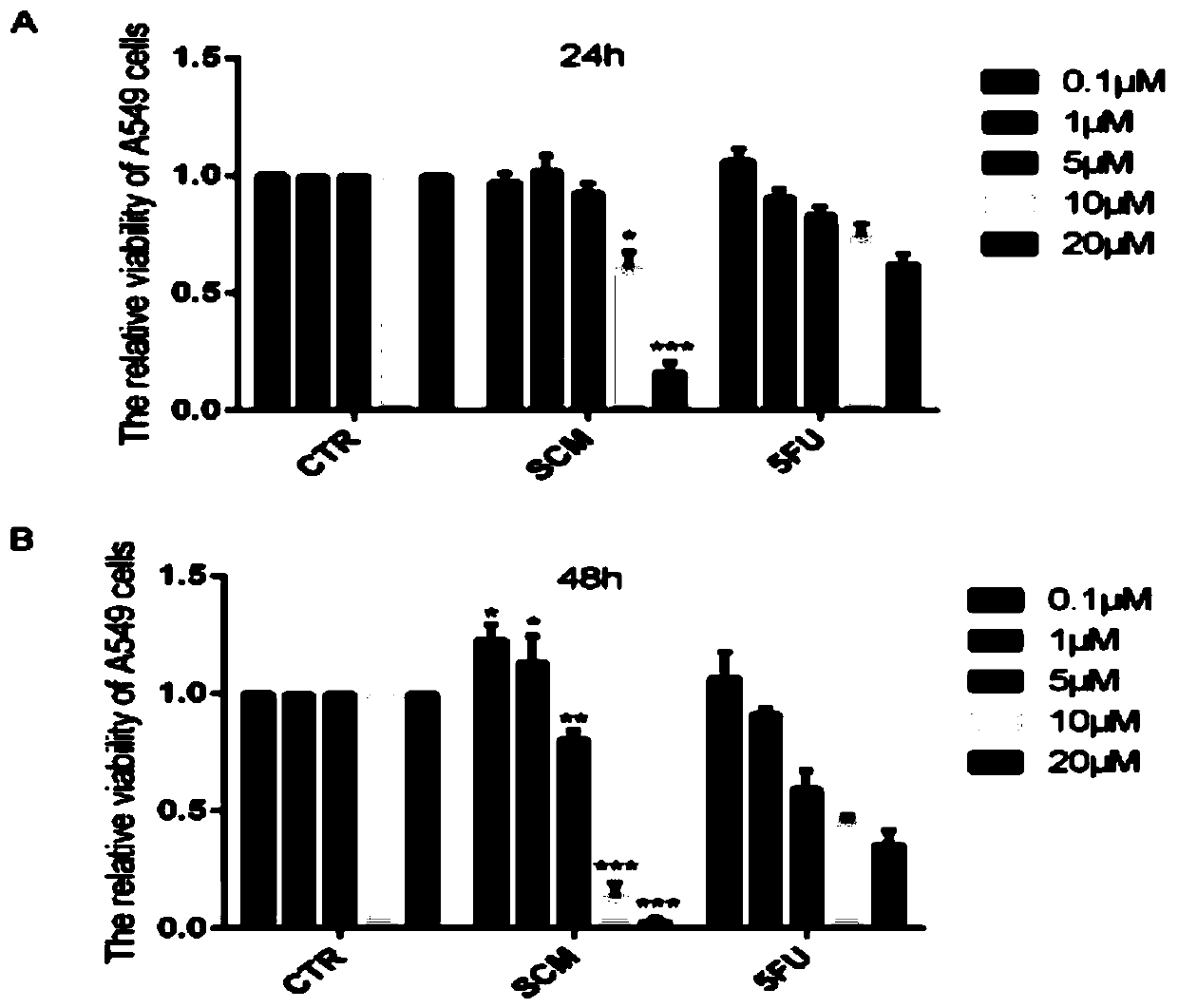
[0037] Example 1: Detection of survival rate by SRB method after SCM treatment of A549 cells.
[0038] (1) The compound SCM was added to treat the cells previously seeded in a 96-well plate. (2) After compound treatment for 24 hours and 48 hours, the old culture medium was discarded, 100 μL of trichloroacetic acid was added, and the cells were fixed at 4° C. for 1 hour. (3) Discard the fixative. Wash five times with double-distilled water in gentle motions, and dry at room temperature. (4) Add 50 μL of SRB to each well, shake at room temperature for 10 minutes. (5) Wash 5 times with 1% acetic acid and dry at room temperature. (6) Add 100 μL Tris base in non-buffer solution with a concentration of 10 mM / L to each well and place on a shaker for 10 minutes. (7) Select 540nm excitation light to measure OD value.
[0039] The results showed that: 5, 10, 20μM SCM treatment of A549 cells for 24h and 48h can significantly inhibit the survival of A549 cells, see figure 1 .
Embodiment 2
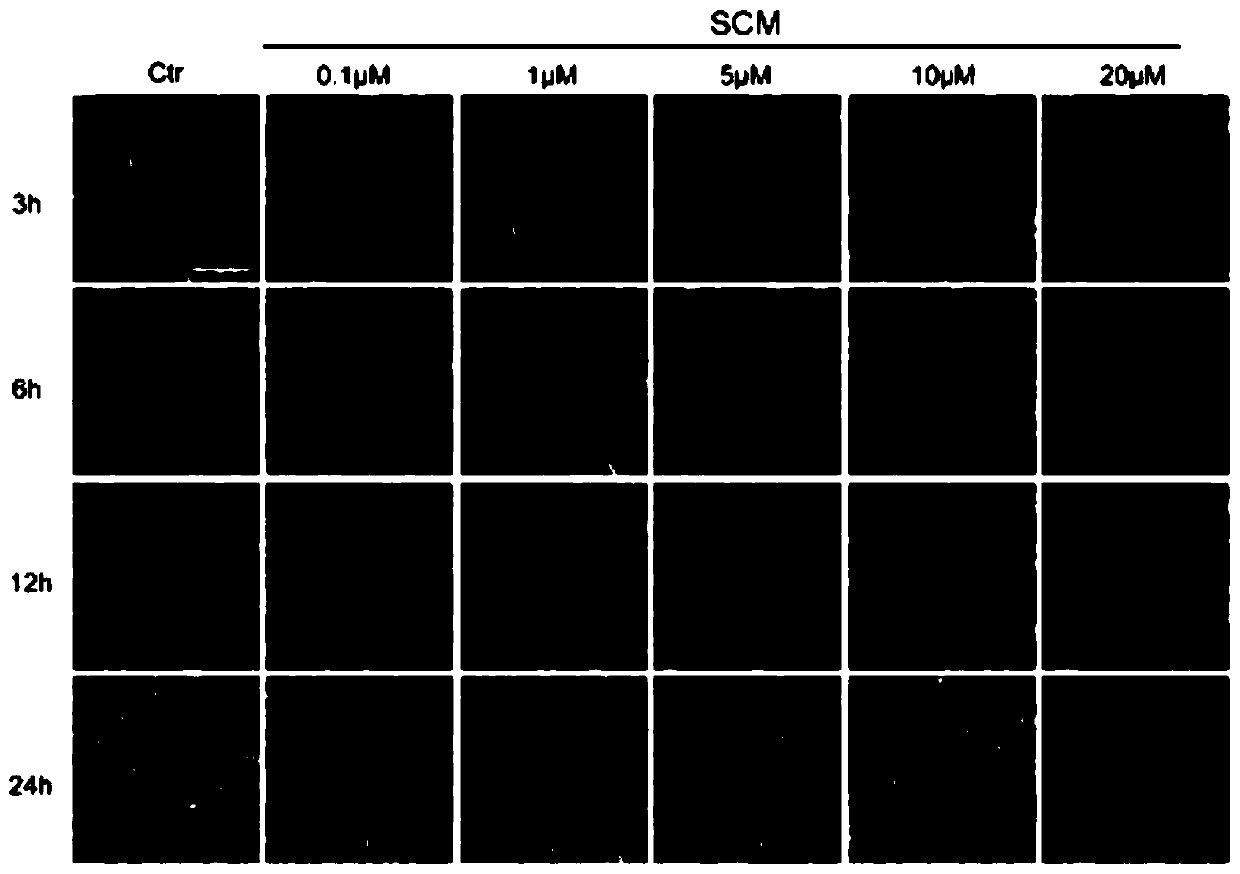
[0040] Example 2: After SCM treatment of A549 cells, the changes in cell morphology were detected under an inverted microscope.
[0041] A549 cells were seeded in a 6 cm-diameter petri dish at 37°C in CO 2 After culturing in the incubator for 12 hours, SCM was added for 3 hours, 6 hours, 12 hours, and 24 hours, and then the cell morphology was photographed under an inverted microscope.
[0042] The results showed that: under the above conditions, SCM could reduce the number of A549 cells and promote the release of apoptotic bodies. See figure 2 .
experiment example 3
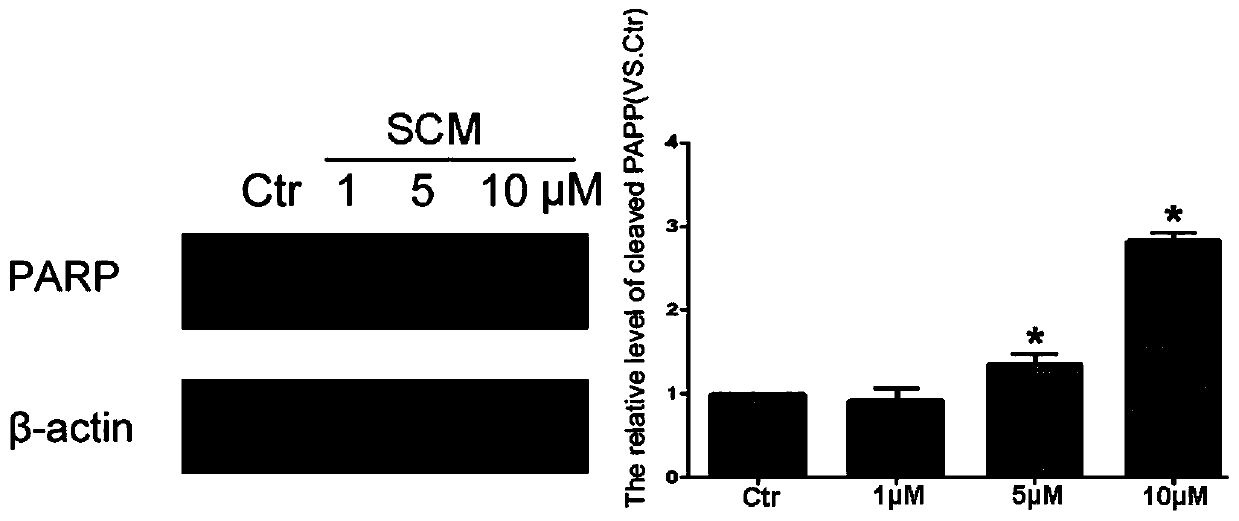
[0043] Experimental Example 3: A549 cells were treated with SCM for 24 hours, and the apoptosis was detected by western blot.
[0044] A549 cells were seeded in a 6 cm-diameter petri dish at 37°C in CO 2 After culturing in the incubator for 12 hours, add SCM for 24 hours, lyse the cells, collect the cell lysate, centrifuge at 12000g for 15 minutes at 4°C, take the supernatant, and use western blot to detect the level of PARP protein cleavage in different cells.
[0045] The results showed that: after treating A549 cells with 1, 5, and 10 μM SCM for 24 hours, they can significantly promote the cleavage of PARP protein, so SCM can significantly promote the apoptosis of A549 cells. See image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com