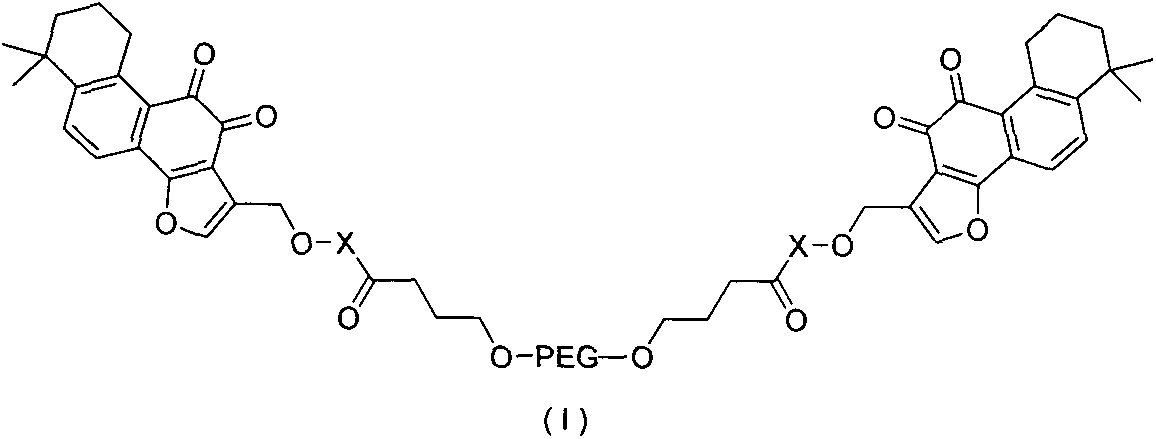
Application of tanshinone IIA derivative in preparation of ischemic stroke drugs
A technology for ischemic stroke and tanshinone, applied in the field of medicine, can solve the problems of low bioavailability, insignificant protective effect of cerebral infarction and cerebral edema, poor water solubility of tanshinone IIA, etc., so as to improve water solubility and increase bioavailability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Primary Screening Experiment of Tanshinone IIA Derivatives
[0018] 1. Test method
[0019] 1. Establishment of the oxygen-glucose deprivation / reoxygenation (OGD / R) model of rat primary cortical neurons
[0020] Sodium dithionite (Na 2 S 2 o 4 ) dry powder fully dissolved, so that the final concentration of 20mM, with NaHCO 3 Adjust the pH to 7.2, which is the anoxic solution. The primary cortical neuron cells were cultured in a 24-well cell culture plate for 7-10 days, and the cells in good growth state were selected for experiments. Before administration, the medium in the 24-well plate was gently aspirated, washed twice with PBS buffer, and then cultured with drug-containing medium. The experiment included a blank control group, a model control group, a tanshinone IIA derivative group, and a tanshinone IIA group. After 24 hours, the drug-containing medium was removed and replaced with anoxic solution, and an equal volume of maintenance medium (Neuro...
Embodiment 2
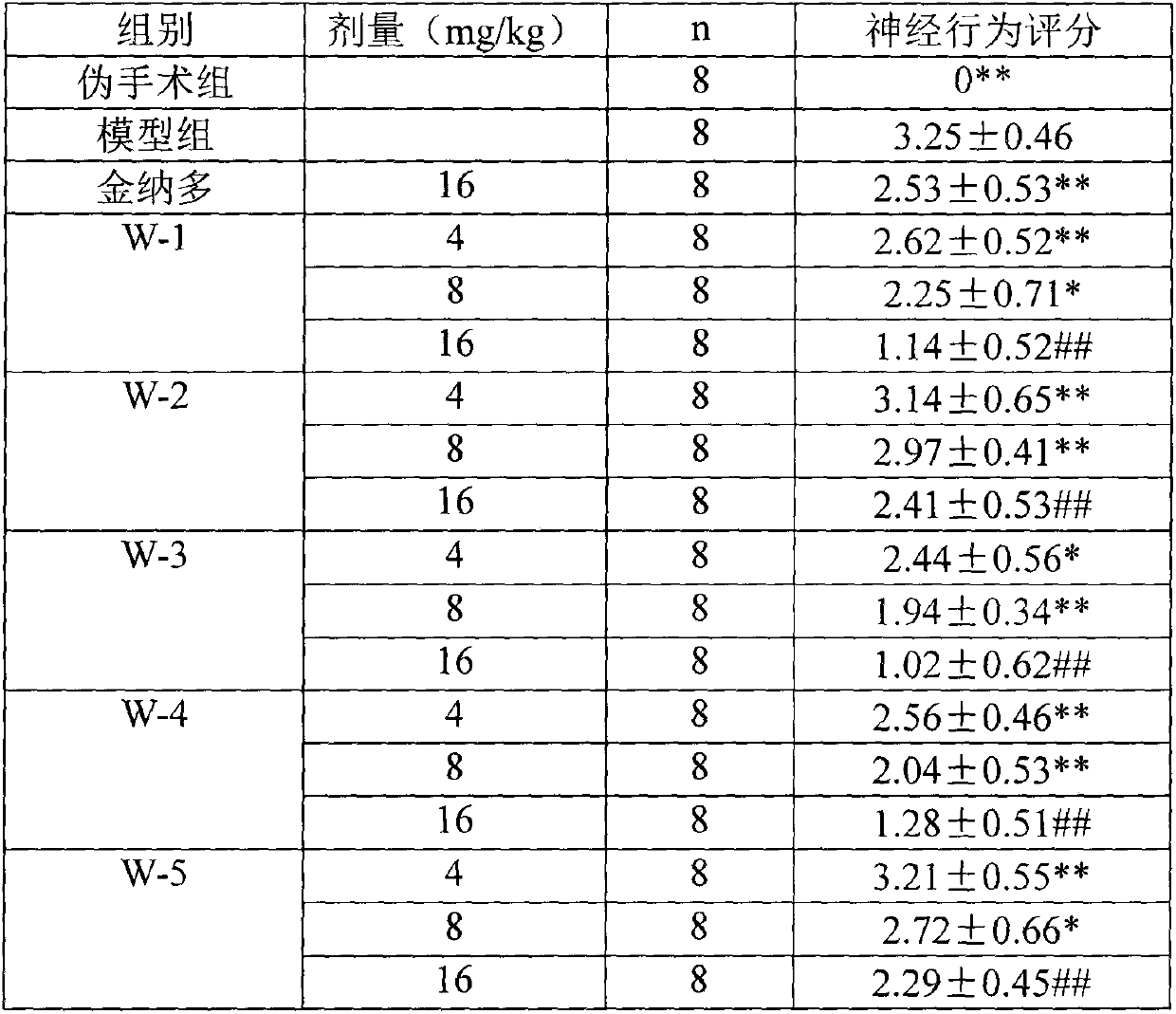
[0028] Example 2: Determination of the protective effect of tanshinone IIA derivatives on cerebral ischemia-reperfusion injury
[0029] 1. Experimental purpose: To establish SD rat MCAO animal model, and to observe the protective effect of test drugs W-1~W-5 on cerebral infarction and cerebral edema caused by cerebral ischemia-reperfusion.
[0030] 2. Experimental method
[0031] 1. Focal cerebral ischemia / reperfusion model in rats
[0032] No food or water was allowed 24 hours before the modeling operation. Referring to the method of Longa et al., the middle cerebral artery occlusion (MCAO) model in rats was prepared by the internal carotid artery suture method: the rats were anesthetized by intraperitoneal injection (ip) of 3% chloral hydrate (300 mg / kg), and operated in supine position. On the stage, make a midline incision in the neck, expose the right common carotid artery, pull the digastric muscle and sternocleidomastoid muscle outwards, free from the bifurcation of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com