Catalytic system for hydrogenation of carbon dioxide and method for synthesizing n-butanol
A catalytic system, carbon dioxide technology, applied in the direction of organic compound/hydride/coordination complex catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve environmental pollution and other problems, achieve easy access to raw materials, and improve reactions Efficiency, good economical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The present embodiment prepares n-butanol by the following method:
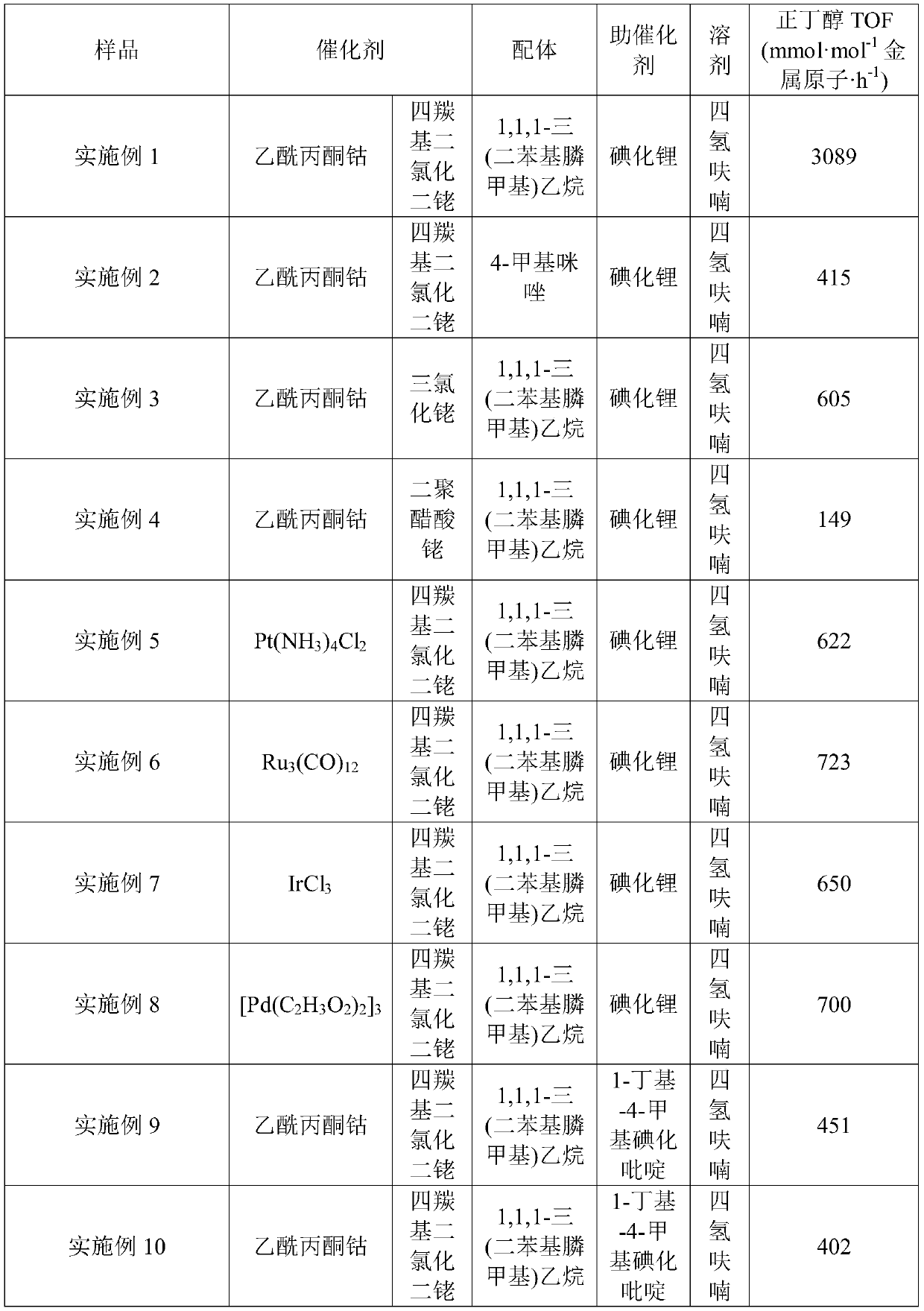
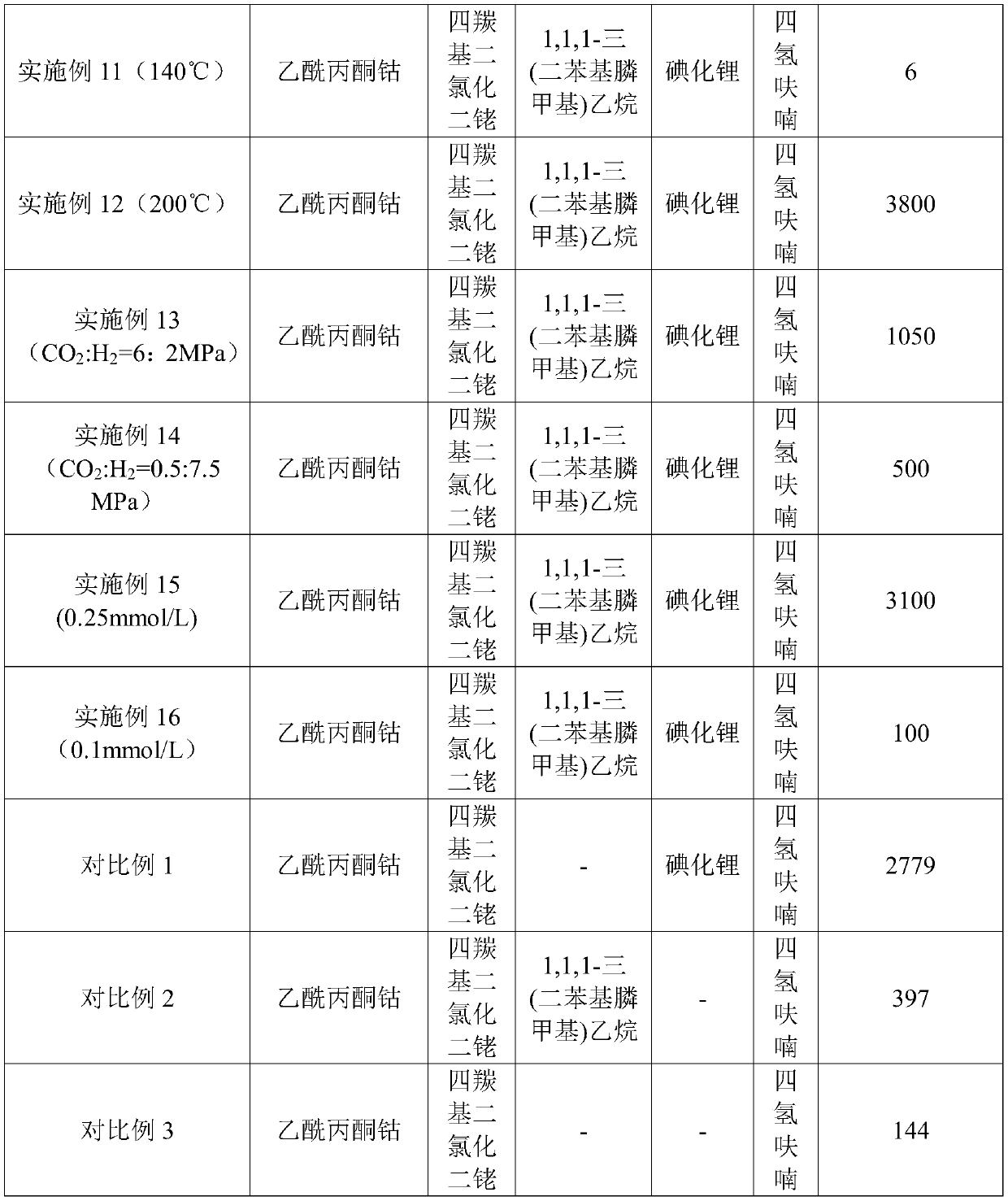
[0040] Add cobalt acetylacetonate, dirhodium tetracarbonyl dichloride, 1,1,1-tris(diphenylphosphinemethyl)ethane, lithium iodide and tetrahydrofuran solvent into a stainless steel reaction kettle with a volume of 50mL (built-in electromagnetic stirring Magneto), seal the reaction kettle, replace it with 2MPa carbon dioxide for 5 times, then fill it with 2MPa carbon dioxide, and then fill it with hydrogen, so that the partial pressure ratio of carbon dioxide and hydrogen is 1:3, and put the reaction kettle into the heating jacket , set the rotating speed to 600 r / min, raise the temperature, and react for 8 hours after reaching 180°C. After the reaction was over, the reactor was cooled, and then the gas was released. The liquid product was analyzed by gas chromatography.
[0041] Concrete catalyst consumption is as follows: cobalt catalyst 14mmol / L, rhodium catalyst 14mmol / L, organophosphine ligand 28m...
Embodiment 2
[0043] The present embodiment prepares n-butanol by the following method:
[0044] Add cobalt acetylacetonate, rhodium tetracarbonyl dichloride, 4-methylimidazole, lithium iodide synergistic catalyst and tetrahydrofuran solvent into a stainless steel reactor (built-in electromagnetic stirring magnet) with a volume of 50mL, seal the reactor, and use 2MPa carbon dioxide was replaced 5 times, then charged with 2MPa carbon dioxide, and then flushed with hydrogen, so that the partial pressure ratio of carbon dioxide and hydrogen was 1:3, and the reaction kettle was placed in the heating jacket, and the speed was set at 600 rpm. The temperature was raised to 180° C. and reacted for 8 hours. After the reaction was over, the reactor was cooled, and then the gas was released. Liquid and gaseous products were analyzed by gas chromatography.
[0045] Concrete catalyst consumption is as follows: cobalt catalyst 14mmol / L, rhodium catalyst 14mmol / L, organophosphine ligand 28mmol / L, iodide...
Embodiment 3
[0047] The present embodiment prepares n-butanol by the following method:
[0048] Add cobalt acetylacetonate, rhodium trichloride, 1,1,1-tris(diphenylphosphinemethyl)ethane, lithium iodide co-catalyst and tetrahydrofuran solvent into a stainless steel reaction vessel with a volume of 50mL (built-in electromagnetic stirring magnetic In the sub), seal the reactor, replace it with 2MPa carbon dioxide for 5 times, then fill it with 2MPa carbon dioxide, and then fill it with hydrogen, so that the partial pressure ratio of carbon dioxide and hydrogen is 1:3, and put the reactor into the heating jacket, Set the rotation speed to 600 rpm, and raise the temperature until it reaches 180°C and react for 8 hours. After the reaction was over, the reactor was cooled, and then the gas was released. The liquid product was analyzed by gas chromatography.
[0049] Concrete catalyst consumption is as follows: cobalt catalyst 14mmol / L, rhodium catalyst 7mmol / L, organic phosphine ligand 28mmol / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com