Glycoprotein multi-charge isomer post-translational modification evaluation method
A technology of post-translational modification and charge isomerization is applied in the field of evaluation of post-translational modification of various charge isoforms of glycoproteins, which can solve the problems of complex modification and achieve the effect of convenient operation and good reference.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
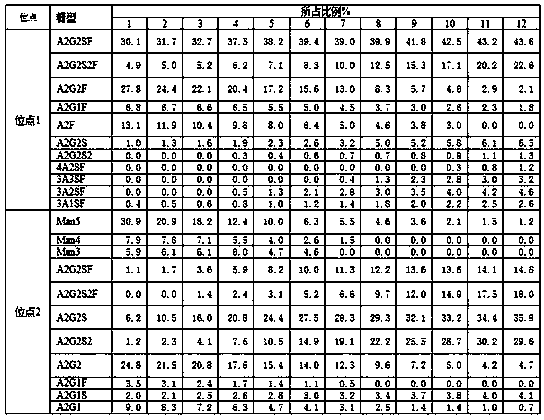
[0022] Example 1. Structural Analysis of Post-translational Modifications of Charge Variant of Recombinant Human VEGFR-Fc Fusion Protein
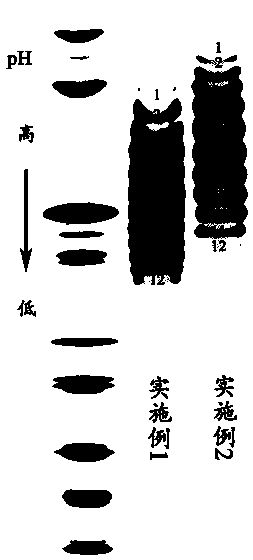
[0023] (1) IEF separation charge heterogeneity
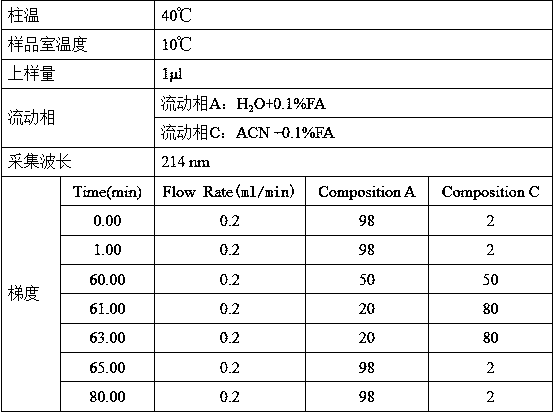
[0024] Take 1mg of recombinant human VEGFR-Fc fusion protein, use 10kD, 0.5ml ultrafiltration tube to replace liquid by ultrafiltration into 100μl ultrapure water, and quantify the protein concentration with a microplate reader. Take an appropriate amount of ultrafiltered sample and mix it with 2×sample buffer in an equal volume before loading the sample. The protein loading amount is 100 μg / band, and the gel used is pH3-10 IEF gel. Electrophoresis conditions were as follows.
[0025] Table 1 IEF electrophoresis voltage settings
[0026]
[0027] After electrophoresis, the gel was rinsed twice with ultrapure water, then soaked in 12% trichloroacetic acid fixative solution for fixation, and shaken at room temperature for 30 min. After washing the gel three times with ultrapure water, add...
Embodiment 2
[0052] Example 2, High pI recombinant human VEGFR-Fc fusion protein charge variant post-translational modification structure analysis
[0053] (1) IEF separation charge heterogeneity
[0054] Take 1mg of high pI recombinant human VEGFR-Fc fusion protein, use 10kD, 0.5ml ultrafiltration tube to replace liquid by ultrafiltration into 100μl ultrapure water, and quantify the protein concentration with a microplate reader. Take an appropriate amount of ultrafiltered sample and mix it with 2×sample buffer in an equal volume before loading the sample. The protein loading amount is 100 μg / band, and the gel used is pH3-10 IEF gel. Electrophoresis conditions were as follows.
[0055] Table 1 IEF electrophoresis voltage settings
[0056]
[0057] After electrophoresis, the gel was rinsed twice with ultrapure water, then soaked in 12% trichloroacetic acid fixative solution for fixation, and shaken at room temperature for 30 min. After washing the gel three times with ultrapure water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com