Preparation method of tryptopquinoline heterocyclic compound
A technology of heterocyclic compounds and quinoline, which is applied in the field of organic synthesis, can solve the problems of no chromonoquinoline heterocyclic compounds and cumbersome preparation methods, and achieve the effects of simple reaction, simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]
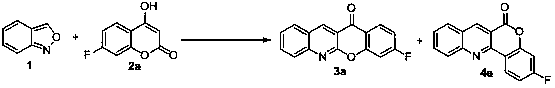
[0022] In a 15 mL tube were added anthranilic anhydride 1 (60 mg, 0.5 mmol) and 4-hydroxycoumarin 2a (0.65 mmol), followed by DMI (0.75 mL); the resulting mixture was then warmed to 230 °C React for 1 hour; stop heating after the reaction is over, cool to room temperature, add water and ethyl acetate, then wash the organic phase three times with water, combine the aqueous phase, extract the aqueous phase once with ethyl acetate, finally combine the organic phase, wash through anhydrous sulfuric acid After sodium drying, the solvent was evaporated under reduced pressure, and the residue was separated and purified by silica gel column chromatography to obtain products 3a and 4a respectively, with a total yield of 69%, 3a:4a=1.4:1. 3a: 1 H NMR (400 MHz, CDCl 3 ) δ: 9.29 (s, 1H), 8.41-8.30 (m, 1H), 8.16-8.03 (m, 2H), 7.92 (t, J =7.6 Hz, 1H), 7.64 (t, J = 7.5 Hz, 1H), 7.30 (d, J = 9.0 Hz, 1H), 7.15 (t, J =7.9 Hz, 1H); 13 C NMR (151 MHz, CDCl 3 ) δ: 177.02, 167...
Embodiment 2
[0024]
[0025] Into a 15 mL tube were added anthranilide 1 (60 mg, 0.5 mmol) and 4-hydroxycoumarin 2a (0.05 mmol), followed by DOA (0.75 mL). The reaction mixture was warmed up to 300 °C for 0.2 hours. Stop heating, cool to room temperature, add water and ethyl acetate. The organic phase was washed three times with water, the aqueous phase was combined, and the aqueous phase was extracted once with ethyl acetate. Finally, the organic phase was combined, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The residue was separated and purified by silica gel column chromatography to obtain the product 3a and 4a, the total yield is 55% (based on 4-hydroxycoumarin), 3a:4a = 1.2:1.
Embodiment 3
[0027]
[0028] Anthranilide 1 (60 mg, 0.5 mmol) and 4-hydroxycoumarin 2a (5 mmol) were added to a 15 mL tube, followed by DMI (5 mL). The reaction mixture was warmed up to 150°C for 5 hours. Stop heating, cool to room temperature, add water and ethyl acetate. The organic phase was washed three times with water, the aqueous phase was combined, and the aqueous phase was extracted once with ethyl acetate. Finally, the organic phase was combined, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The residue was separated and purified by silica gel column chromatography to obtain the product 3a and 4a, the total yield is 63%, 3a:4a = 1.3:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com