Synthesis method of Daclatasvir
A synthesis method and technology of synthesis process, applied in the direction of organic chemistry, etc., can solve the problems of unsustainable response of patients, cumbersome treatment, adverse reactions, etc., and achieve the effects of cheap raw materials, simplified operation process, and little environmental pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
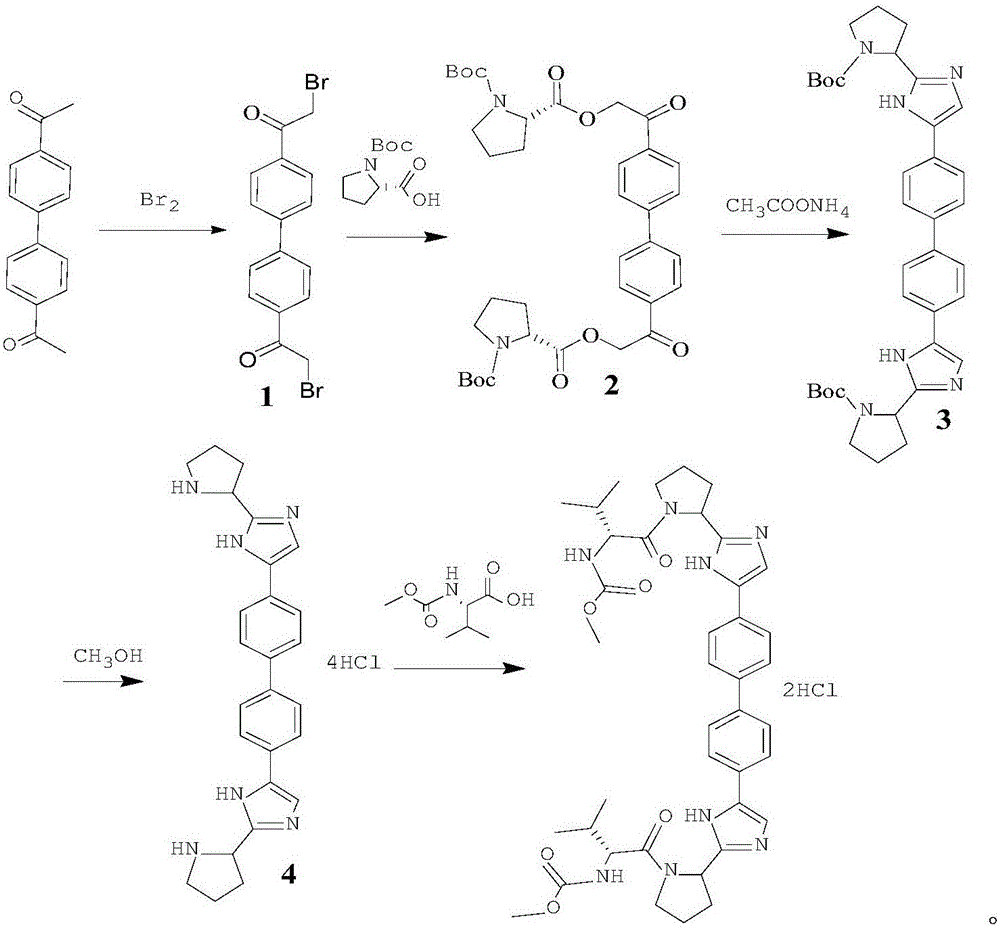
[0030] This embodiment relates to a synthetic method of daclatasvir, which consists of the following steps:
[0031] Step 1, the synthesis of intermediate 1
[0032] Dissolve 4.4-diacetylbiphenyl (40g, 0.17mol) and DIPEA (53g, 0.41mol) in 1000mL of dichloromethane, add TMSOTf (0.37mol) dropwise at 0°C, stir for 15 minutes after dropping, add NBS ( 0.39mol) of dichloromethane (200mL) solution, after dripping, stirred and reacted at 0°C for 2h, the reaction was complete, the system was poured into 500mL of water, layered, dried over anhydrous sodium sulfate, filtered with suction, concentrated to remove dichloromethane to obtain the intermediate 1 crude product, crystallized from toluene to obtain 61g solid, yield 91%;
[0033] MP: 224-226°C, 1 HNMR (400MHz, CDCl 3 ):δ(ppm):7.96-7.84(m,4H),7.61-7.52(m,4H),4.26(s,4H), HRMS(ESI):m / z[M+H] - 394.93.
[0034] Step 2, the synthesis of intermediate 2
[0035] Intermediate 1 (40g, 0.101mol) and L-proline derivative (60g, 0.222mol)...
Embodiment 2
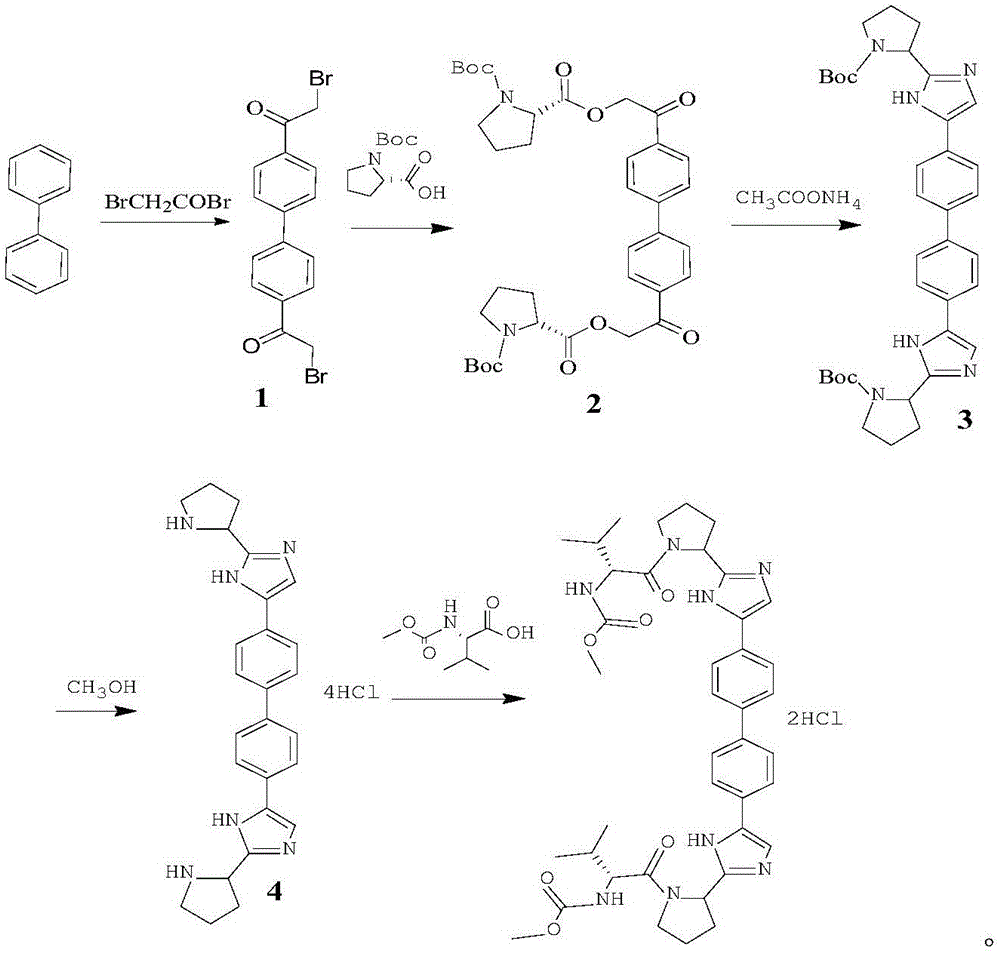
[0040] This embodiment relates to a synthetic method of daclatasvir, which consists of the following steps:
[0041] Step 1, the synthesis of intermediate 1
[0042]Dissolve 4.4-diacetylbiphenyl (40g, 0.17mol) and DIPEA (49g, 0.38mol) in 1000mL of dichloromethane, add TMSOTf (0.39mol) dropwise at 0°C, stir for 15 minutes after dropping, add NBS ( 73g, 0.41mol) of dichloromethane (200mL) solution, after dripping, stirred and reacted at 0°C for 2h, the reaction was complete, the system was poured into 500mL of water, layered, dried over anhydrous sodium sulfate, suction filtered, concentrated to remove dichloromethane, to obtain The crude intermediate 1 was crystallized from toluene to obtain 62 g of solid, with a yield of 92%;
[0043] MP:224.5-226.5℃, 1 HNMR (400MHz, CDCl 3 ):δ(ppm):7.95-7.84(m,4H),7.62-7.52(m,4H),4.27(s,4H), HRMS(ESI):m / z[M+H] - 394.94.
[0044] Step 2, the synthesis of intermediate 2
[0045] Dissolve Intermediate 1 (40g, 0.101mol) and L-proline deriva...
Embodiment 3
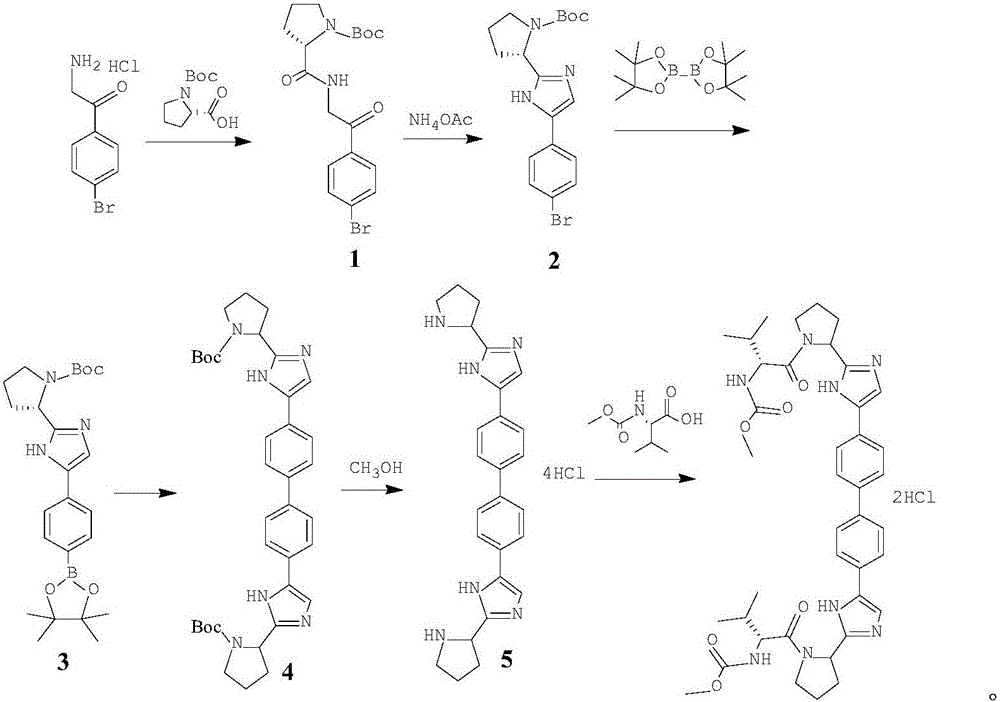
[0050] This embodiment relates to a synthetic method of daclatasvir, which consists of the following steps:
[0051] Step 1, the synthesis of intermediate 1
[0052] Dissolve 4.4-diacetylbiphenyl (40g, 0.17mol) and DIPEA (0.39mol) in 1000mL of dichloromethane, add TMSOTf (91g, 0.41mol) dropwise at 0°C, stir for 15 minutes after dropping, add NBS ( 66g, 0.37mol) of dichloromethane (200mL) solution, after dripping, stirred and reacted at 0°C for 2h, the reaction was complete, the system was poured into 500mL water, layered, dried over anhydrous sodium sulfate, suction filtered, concentrated to remove dichloromethane, and obtained The crude intermediate 1 was crystallized from toluene to obtain 62 g of solid, with a yield of 92%;
[0053] MP: 225-226°C, 1 HNMR (400MHz, CDCl 3 ):δ(ppm):7.96-7.85(m,4H),7.60-7.52(m,4H),4.27(s,4H), HRMS(ESI):m / z[M+H] - 394.93.
[0054] Step 2, the synthesis of intermediate 2
[0055] Intermediate 1 (40g, 0.101mol) and L-proline derivative (60g,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com