CGTase high in temperature stability and gamma-cyclodextrin prepared from CGTase
A cyclodextrin and maltodextrin technology, applied in the directions of transferase, fermentation, etc., can solve the problems of residue, low conversion rate, poor temperature stability, etc., and achieve the effect of reducing use and improving starch conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
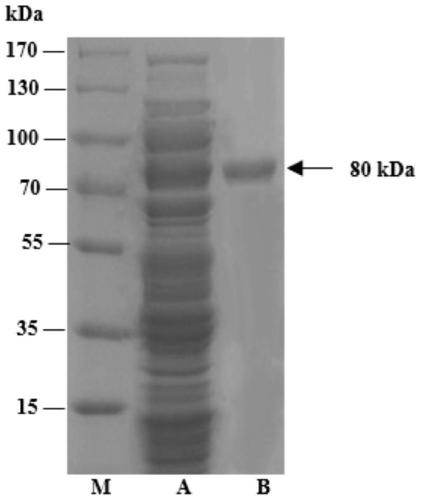
[0031] Embodiment 1: the preparation of enzyme
[0032] The inventors conducted a lot of research and experiments, and unexpectedly found a gene annotated as α-amylase on NCBI (its amino acid sequence is shown in SEQ ID NO.1), and its expression product has the function of synthesizing γ-CD. Under normal circumstances, the general α-amylase can only hydrolyze the α-1,4-glucosidic bond in the starch molecular chain, and does not have the function of catalyzing the synthesis of γ-CD from starch.
[0033] The gene fragment whose nucleotide sequence is shown in SEQ ID NO.2 (amino acid sequence is shown in SEQ ID NO.1) was coded and synthesized at Beijing Huada Gene Co., Ltd., using pET-15b as a plasmid, NcoI and XhoI as enzymes site, connect the gene sequence to the plasmid, and transfer it to E.coli BL21(DE3) for expression. Place E.coli BL21(DE3) in LB broth medium containing 100 μg / mL ampicillin, and culture it on a shaker at 37°C and 200r / min until OD 600 Reach 0.4 to 0.6. ...
Embodiment 2
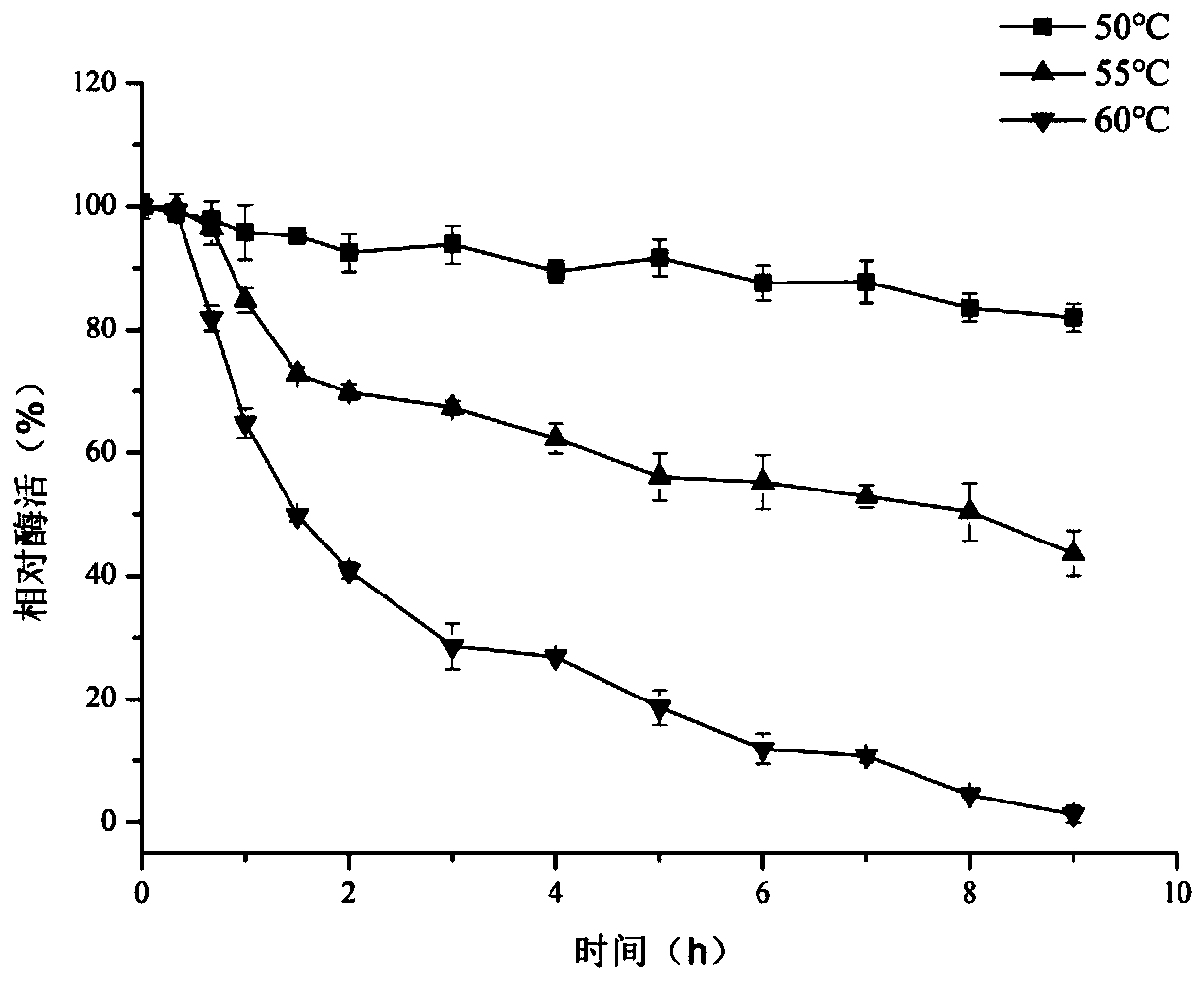
[0034] Example 2: Temperature Stability of Enzymes
[0035] Add Ca at a final concentration of 5mmol / L 2+ To the enzyme purified in Example 1, incubate at different temperatures of 50, 55, and 60° C. for 0 min, 20 min, 40 min, 60 min, 90 min, 2 h, 3 h, 4 h, 5 h, 6 h, 7 h, 8 h, and 9 h. The residual cyclodextrin glucosyltransferase activity of γ-CGTase was measured at different temperatures and different incubation times.
[0036] The results show that (see figure 2 ): The enzyme has a residual enzyme activity of 80% after incubation at 50°C for 9 hours, a half-life of 8 hours at 55°C, and a half-life of 90 minutes at 60°C, indicating that it has good temperature stability.
Embodiment 3
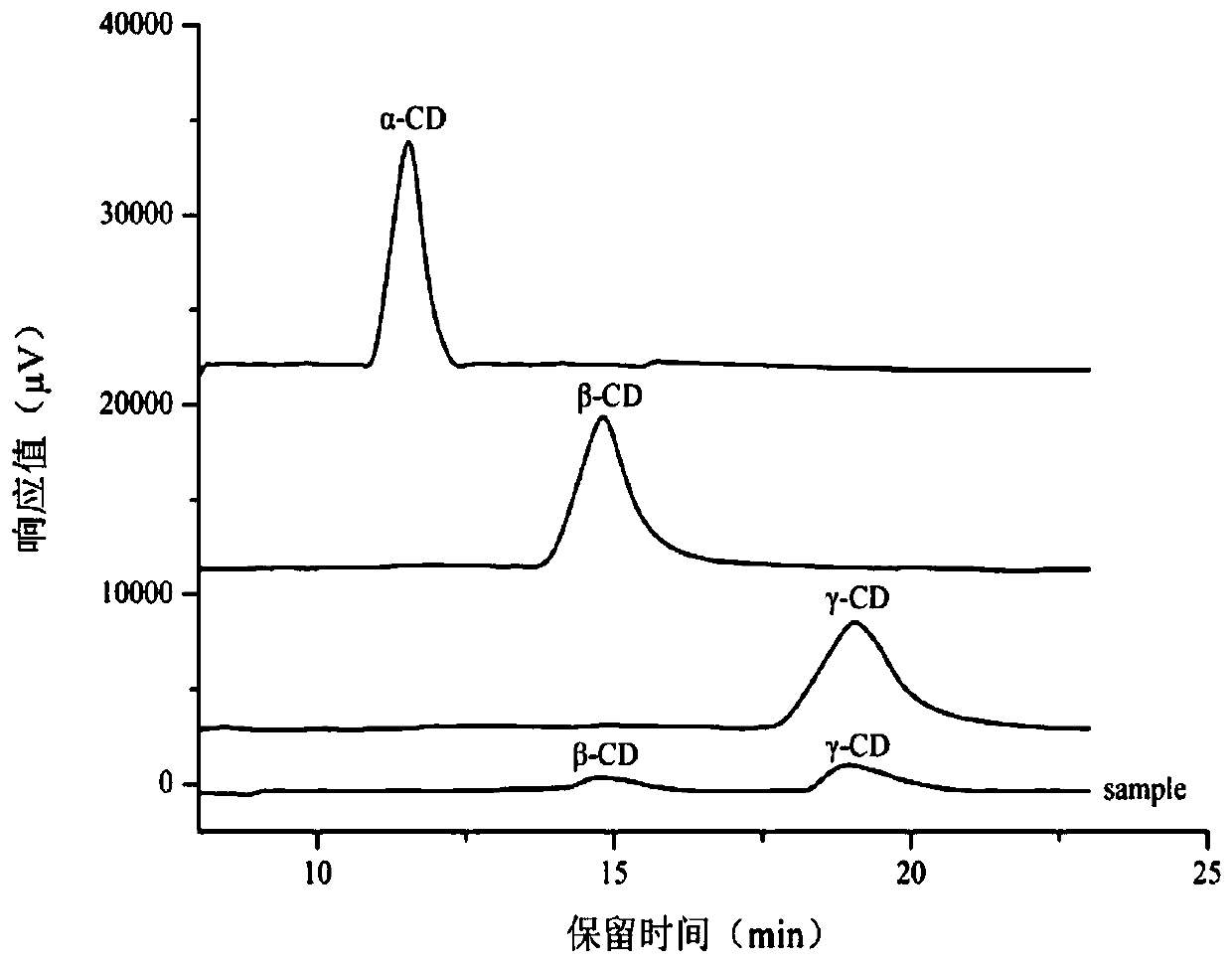
[0037] Embodiment 3: Preparation of γ-CD
[0038] (1) Mix 15 mg of soluble starch solution with 1 mL of distilled water (1.5% substrate concentration), and stir for 15 min at 90°C;
[0039] (2) Lower the temperature to 50°C, adjust the pH to 9.0, add 0.05U of the enzyme purified in Example 1, react for 0.5h, then boil in a water bath at a high temperature of 100°C for 10min to kill the enzyme, filter at normal pressure, evaporate and concentrate, and Dry to obtain γ-cyclodextrin. The conversion of γ-cyclodextrin was measured to be 10%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com