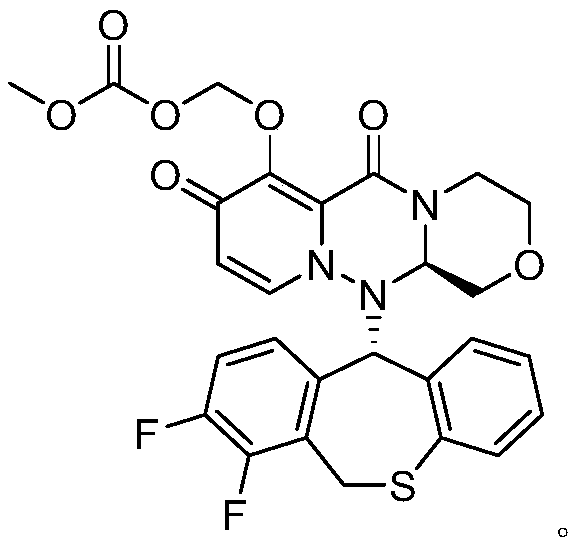
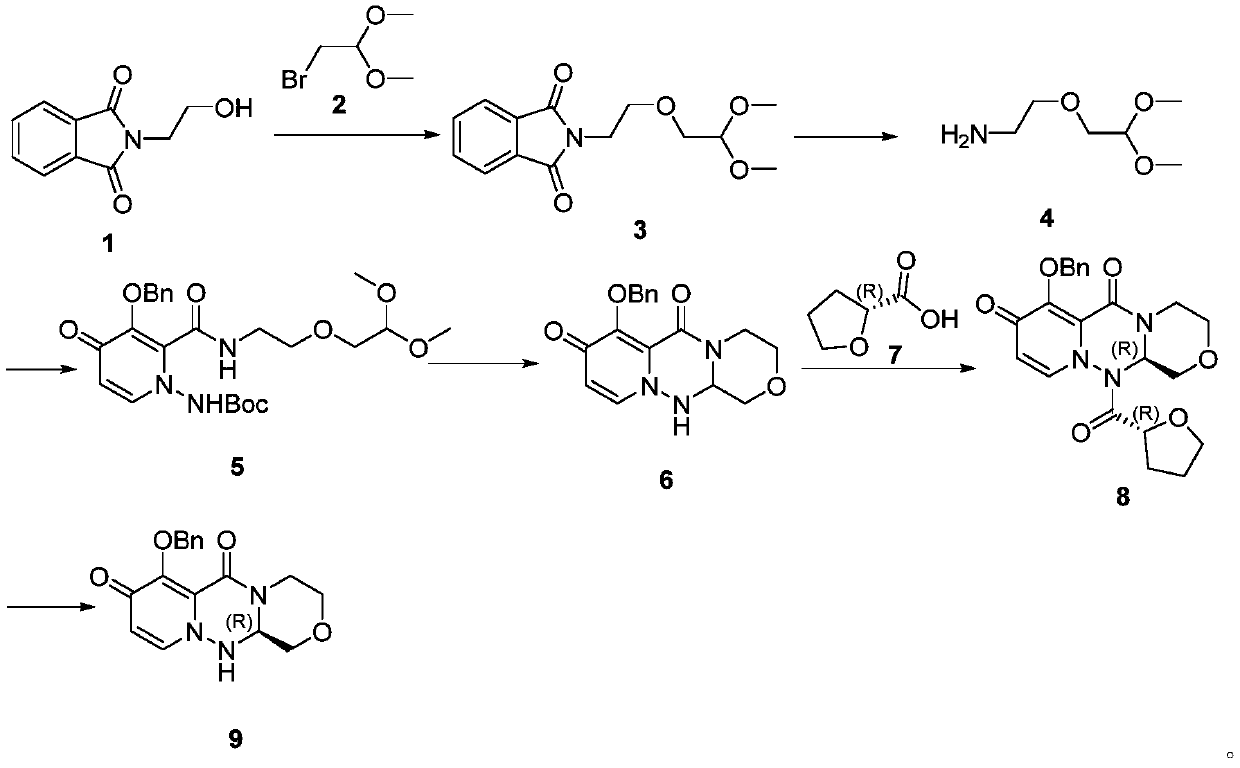
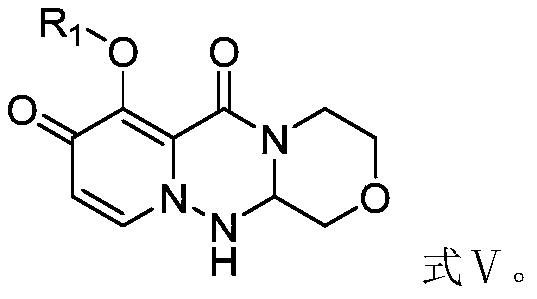
A kind of preparation method of cyclopyridone compound
A kind of technology of cyclopyridone and compound, applied in the field of preparation of cyclopyridone compound, can solve the problems of complicated post-processing operation, large loss, long operation steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] The preparation of embodiment 1 formula II compound (LG is a methoxyl group)
[0081] Add compound formula I (10.0g) and dichloromethane (100mL) into the reaction bottle, stir at 0°C, then add methyl trifluoromethanesulfonate (19.48g) dropwise, after the addition is complete, rise to room temperature and stir, react After completion, the reaction was quenched with aqueous sodium carbonate solution, extracted with dichloromethane, and the solvent was evaporated to dryness under reduced pressure to obtain 7.51 g of the compound of formula II with a yield of 66%.
Embodiment 2
[0082] The preparation of embodiment 2 formula IV compound (R 1 is benzyl, R 2 for methyl)
[0083] Add formula II (12g), formula III compound (10.8g), propylphosphoric anhydride (50% ethyl acetate solution) (70g) into the reaction flask, stir and react at 60°C; after the reaction is complete, add 500mL of water and stir, filter, 10.5 g of the compound of formula IV was obtained with a yield of 85% and a purity of 98%.
[0084] LC-MS: [M+1]=326.31;
[0085] 1 H NMR (400MHz, DMSO) δ8.06(d, J=7.8Hz, 1H), 7.59(d, J=7.2Hz, 2H), 7.35(dt, J=24.8, 7.1Hz, 3H), 6.56(d , J=7.8Hz, 1H), 5.12(s, 2H), 4.52(s, 2H), 4.03(t, J=5.4Hz, 2H), 3.72(t, J=5.4Hz, 2H).
Embodiment 3
[0086] The preparation of embodiment 3 formula II compound (LG is chlorine)
[0087]Add the compound formula I (3.0 g) into the reaction flask, add 20 ml of phosphorus oxychloride, raise the temperature to 80° C. for reaction after the addition, and evaporate the phosphorus oxychloride under reduced pressure to obtain the compound of formula II. Yield 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com