New compound and application thereof in preparation of bacterial inhibitors
A technology of bacterial inhibitors and compounds, applied in the field of new compounds and their application in the preparation of bacterial inhibitors, which can solve the problems of affecting applications, high myocardial toxicity, and strong killing effect on normal cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, acquisition, identification and preservation of bacterial strains
[0031] 1. Acquisition of strains
[0032] 1. Get the disease-free fresh leaves of Tripterygium wilfordii plant in Yao County, Dali, Yunnan Province, and carry out surface disinfection. Surface disinfection method: rinse with clean water, rinse 4 times with clean water, rinse with 75% alcohol for 30s, then soak in 5% sodium hypochlorite for 2s, rinse with sterile water 10 times.
[0033] 2. Sterile filter paper absorbs the moisture on the surface, cuts off the liquid-contact side with a sterilized blade, then cuts the inside into small pieces and transplants it into the modified Martin solid medium containing 150mg / L penicillin and 120mg / L streptomycin , and then pick the growing single colonies for culture.
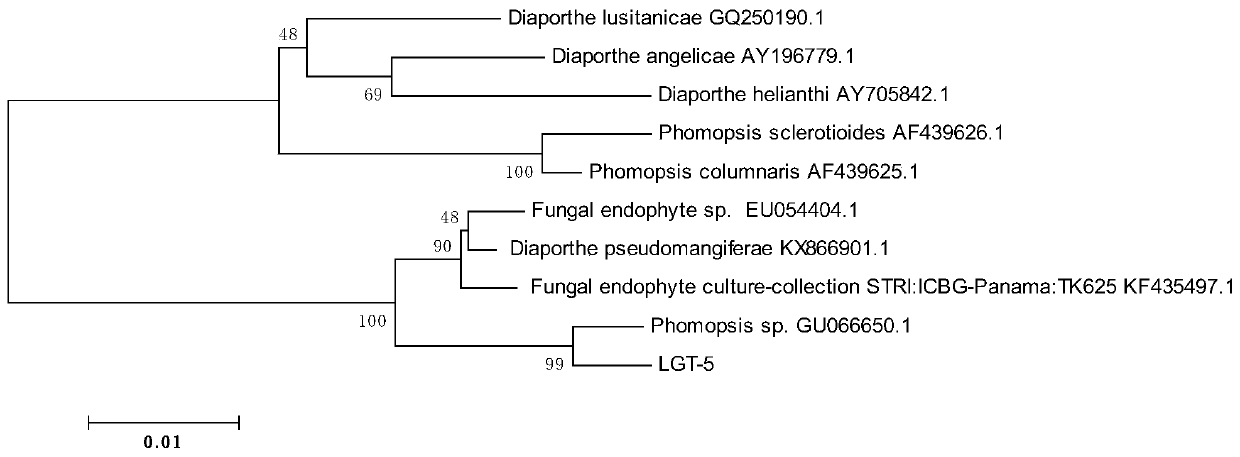
[0034] 3. Further carry out continuous purification to obtain a purely cultured strain, which is named LGT-5 strain.
[0035] 2. Identification of strains
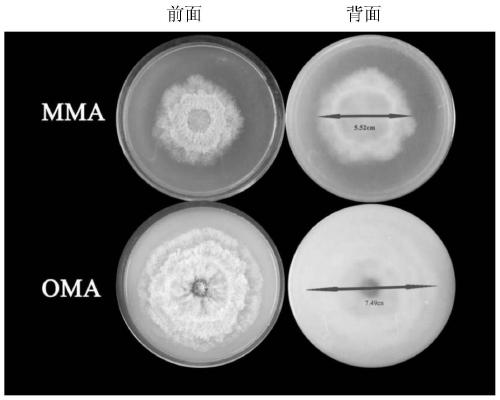
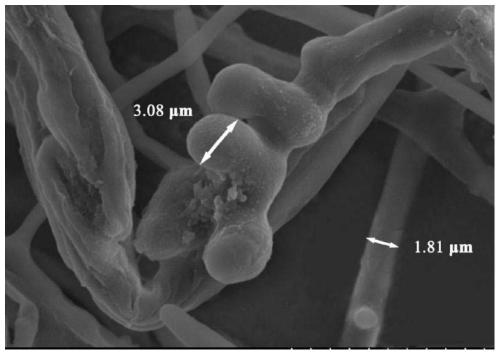
[0036] 1. Colony morph...
Embodiment 2
[0051] Embodiment 2, application Phomopsis LGT-5 prepares 16-OH-Celastrol
[0052] 1. Application of Phomopsis LGT-5 for tripterine transformation
[0053]1. Inoculate Phomopsis LGT-5 into a Erlenmeyer flask containing 150mL of modified Martin liquid medium, and cultivate it with shaking at 28°C and 180rpm until the logarithmic growth phase (usually the culture time is 1 day), at this time Phomopsis The concentration of mold LGT-5 was 5 g dry weight / L culture system. Dry weight measurement method: sample the culture system, collect the bacteria by centrifugation, wash thoroughly with sterile water, and then dry to dry weight.
[0054] 2. After completing step 1, add 2 mL of tripterine solution (containing 50 mg of tripterine, the solvent is DMSO) to the culture system, and culture with shaking at 28° C. and 180 rpm for 5 days. That is, the ratio of Phomopsis LGT-5 and tripterine is: 0.75 g dry weight of Phomopsis LGT-5: 50 mg tripterine.
[0055] 3. After completing step 2,...
Embodiment 3、1
[0066] Example 3, Functional Verification of 16-OH-Celastrol
[0067] Test compound: Celastrol or 16-OH-Celastrol prepared in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com