Chiral cyclen compounds and their uses
A kind of anodine, chiral technology, applied in the field of chiral anodine compound and its application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
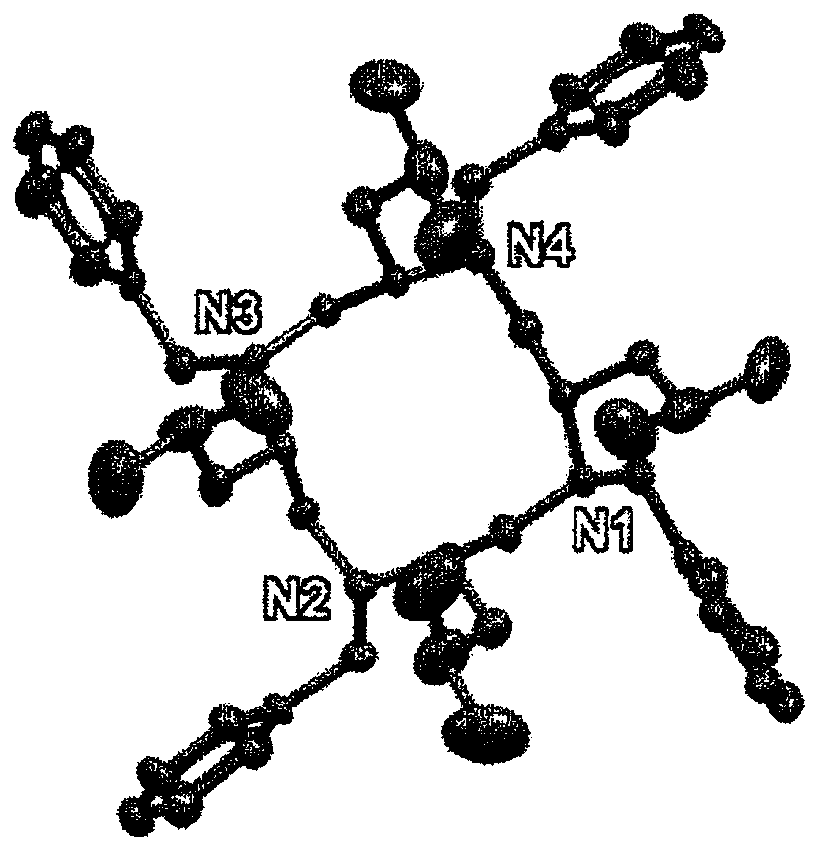
[0341] Synthesis of chiral DOTA metal complexes
[0342] 1. Synthesis of chiral DOTA-based ligands L1-L4
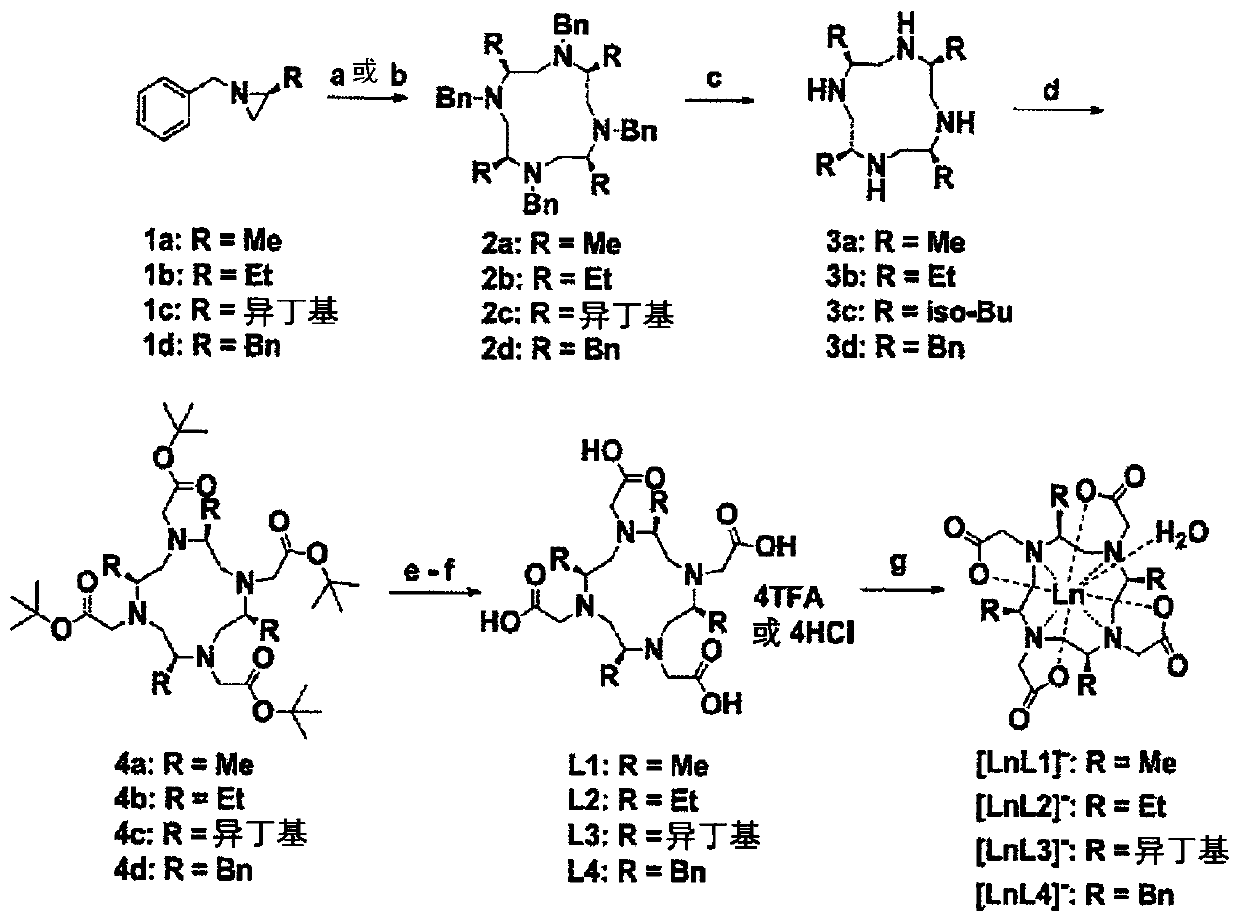
[0343] The synthetic route to prepare chiral DOTA-based ligands is as follows Figure 1A shown. Chiral rhodophonines (3a-3d) from aziridines 1a-1d in the presence of TsOH (step (a)) or Lewis acids (step (b), boron trifluoride and diethyl ether complexes) Synthesize. The cyclized tetrameric products 2a-2d were purified by column chromatography or recrystallization. In step (c), deprotection of the four benzyl groups in 2a-2d yields chiral rotenin 3a-3d. In step (d), the chiral tertenin 3a-3d is reacted with tert-butyl 2-bromoacetate to obtain the chiral tertenin 4a-4d. In steps (e) and (f), the tert-butyl group in the chiral tertenin 4a-4d was deprotected by acid to give DOTA-based ligands L1-L4.
[0344] 2. Synthesis of chiral DOTA-based metal complexes from L1-L4
[0345] exist Figure 1A In step (g), according to [LnDOTA] known in the art - The classical pr...
Embodiment 2
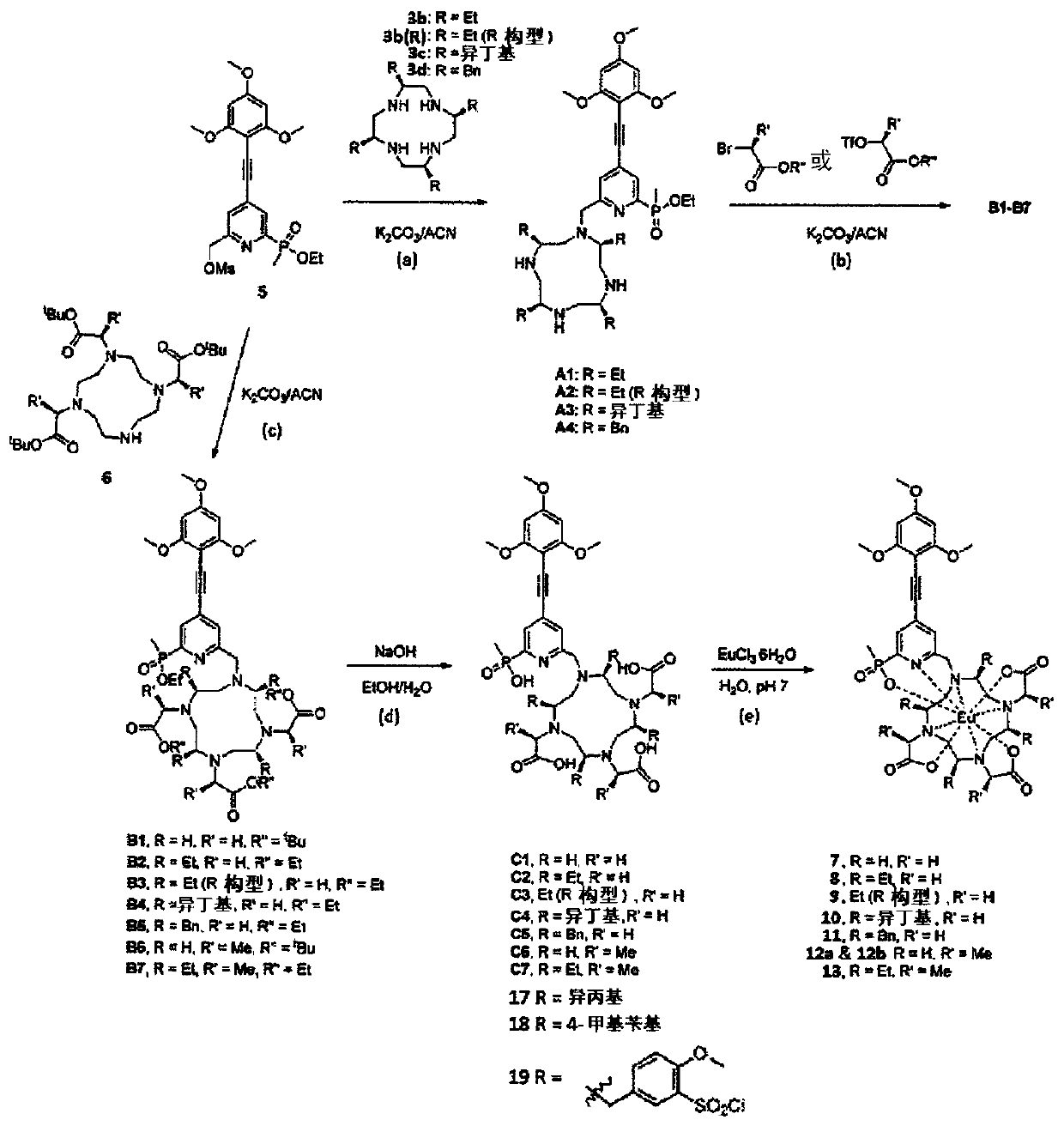
[0429] Synthesis of Metal Complexes Based on Chiral DO3A
[0430] 1. Materials and General Methods.
[0431] Unless otherwise stated, all chemicals were of reagent grade, purchased from Sigma-Aldrich or Acros Organics, which were used without further purification. Humidity-sensitive synthetic procedures were performed under nitrogen atmosphere using standard Schlenk techniques. Davisil silica gel (40-63m) was obtained from Grace Davison. Using analytical reagent grade solvents, acetonitrile was dried over calcium hydride and distilled under nitrogen. High performance liquid chromatography (HPLC) was performed by a Vision HT C18HL 5 mm column using an Agilent 1100 series device with a UV visible detector (UV detection range 220 to 350 nm). Use Waters PrepC18 5m OBDTM (19x 250mm) or (19x 100mm) column for reverse phase semi-preparative purification on an Agilent HPLC system (UV detection range 220 to 360nm). Recorded on a Bruker Ultrashield 400Plus NMR spectrometer (at...
Embodiment 3
[0483] Formation of Copper Complexes Based on Chiral DOTA
[0484] synthesis:
[0485] Chiral DOTA-based lithium salts 29-c and 29-d were synthesized according to similar reaction conditions as described in Examples 1 and 2 ( Figure 29 ).
[0486] LC-MS analysis
[0487] method:
[0488] LC-MS conditions: column: Agilent Eclipse Plus C18RRHD 1.8 μm column (50×2.1 mm), eluent A: water containing 0.01% formic acid, eluent B: acetonitrile.
[0489] [260] Gradient: 2% B, 0-2 minutes; 2-50% B, 2-15 minutes.
[0490] Experimental procedure:
[0491] (1) Inject lithium salt 29-c for analysis.
[0492] (2) Dissolve lithium salt 29-c (7.8 mg, 1.0 eq) in 3 ml NaOAc buffer (1.0 M, pH 5.50), then add CuCl 2 (1.94 mg, 1.0 equiv).
[0493] (3) After the following 5 conditions, 20 μL of the reaction mixture was taken, then diluted with 0.5 mL of NaOAc buffer, and then 5 μL was injected for analysis.
[0494] a) Add CuCl 2 After that, just stir for a few seconds;
[0495] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com