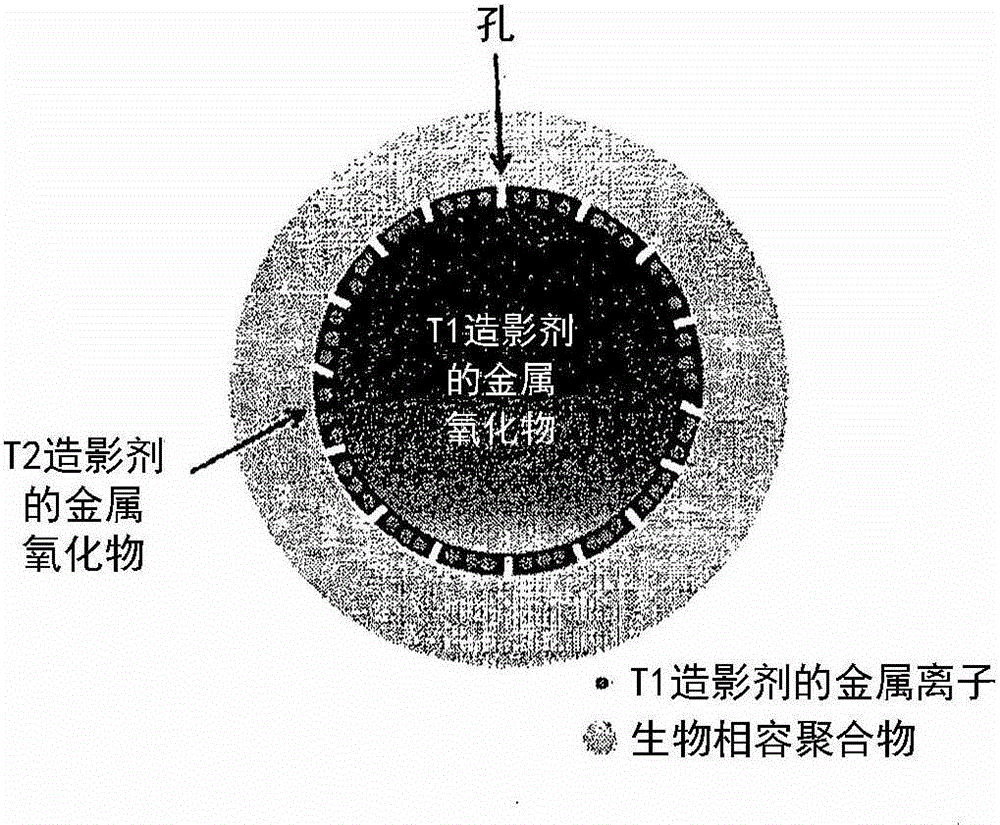
Metal oxide nanoparticle-based t1-t2 dual-mode magnetic resonance imaging contrast agent
A technology of T1-T2 and nanoparticles, which is applied in the fields of nuclear magnetic resonance/magnetic resonance imaging contrast agent, X-ray contrast agent preparation, manganese oxide/manganese hydroxide, etc., can solve the problem that the T1-T2 dual-mode MRI contrast agent has not been developed and other issues to achieve the effect of increasing image contrast enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0054] Example 1: Preparation of Manganese(II) Oxide Nanoparticles of Various Shapes
[0055] Preparation of octahedral manganese oxide nanoparticles
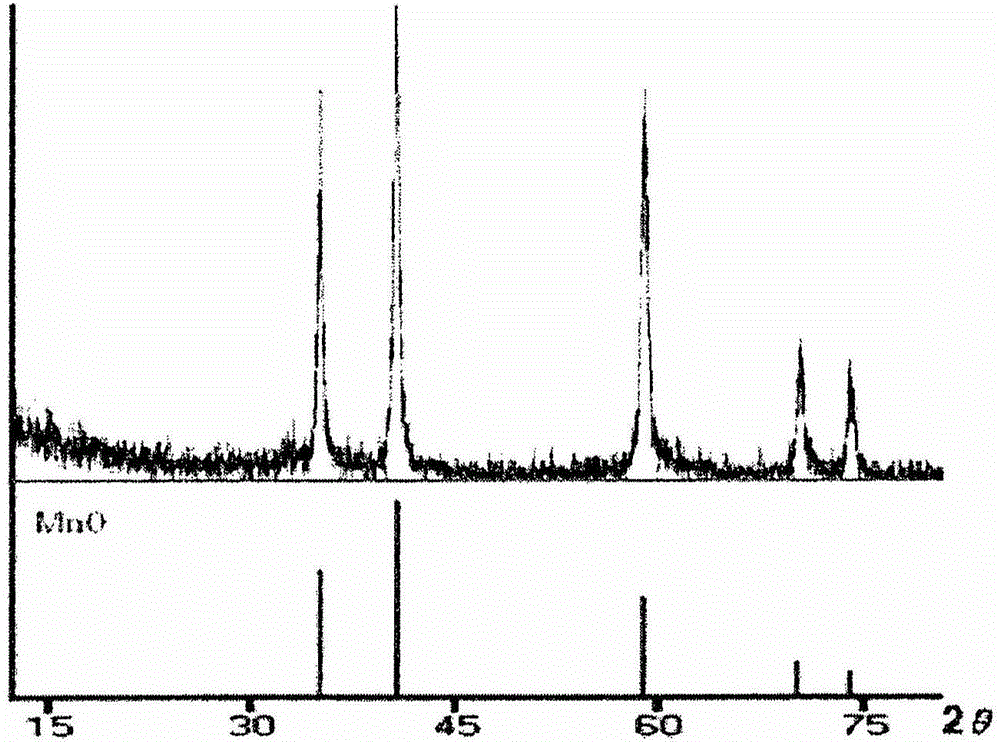
[0056] Octahedral manganese(II) oxide nanoparticles were synthesized by using a reported method (Chem. Mater. 18:1821, 2006) with some modifications. Briefly, manganese(II) formate (Mn(HCOO) 2 , 5mmol), oleic acid (13mmol) and trioctylamine (15mmol) were mixed in a 50ml round bottom flask. Under a strong flow of argon, the mixture was heated to 120° C. in an oil bath with magnetic stirring and kept at this temperature for 3 hours. The temperature was then increased to 330°C at a heating rate of 30°C per minute and the reaction was held at that temperature until a green color appeared. A green solid was obtained by cooling the reaction solution to room temperature, and washing with 1-propanol, followed by centrifugation (3 min, 3,500 rpm). The collected solid was washed again with ethanol several times and then dried ov...
example 2
[0063] Example 2: Preparation of iron oxide nanoparticles with a central MnO phase
[0064] Preparation of iron oxide nanoparticles with central MnO core using octahedral manganese oxide nanoparticles particles
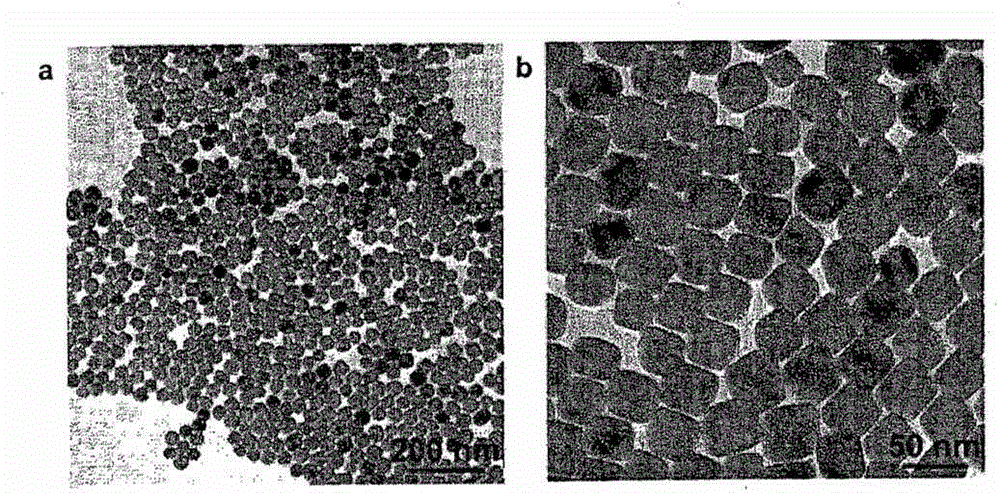
[0065] 14.2 mg of octahedral MnO nanoparticles and 0.375 mmol of iron(III) acetylacetonate were added to a solution of oleic acid (0.05 mmol), oleylamine (1 mmol) and trioctylamine (2 ml) in a 100 ml Schlenk tube. The Schlenk tube was heated in an oil bath under argon at a heating rate of 10 °C / min to 210 °C under vigorous stirring and held at this temperature for 20 min. The reaction mixture was then heated at 310° C. for 30 min in a dry air environment (oxygen percentage 20%). The black solution was cooled to room temperature. After cooling to room temperature, with the addition of acetone and n-propanol, iron oxide nanoparticles with a central MnO phase core precipitated and were collected by centrifugation (3 min, 3,500 rpm). The obtained nanoparticles w...
example 3
[0071] Example 3: Preparation of pyrenyl polyethylene glycol (pyrenyl PEG)
[0072] By making heterofunctionalized polyethylene glycol (NH 2 -PEGCOOH, molecular weight: 5,000Da) combined with the n-hydroxysuccinimide (NHS) group of 1-pyrene butyric acid n-hydroxysuccinimide ester (Py-NHS, molecular weight: 385.41Da) to synthesize pyrene-based polymers. Ethylene glycol (pyrenyl PEG). In detail, 3 mmol of Py-NHS and 1 mmol of NH 2 -PEG-COOH was dissolved in 15 ml dimethylformamide, and then 200 μl triethylamine was added to the reaction mixture at room temperature. After reacting for 48 hours at room temperature under a nitrogen atmosphere, the resulting product was filtered and washed with excess ether. The precipitate was dried under vacuum and stored for later use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com