A kind of aurora A protein inhibitor and its preparation method and pharmaceutical application
A kind of use and medicine technology, applied in the field of AuroraA protein inhibitor and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
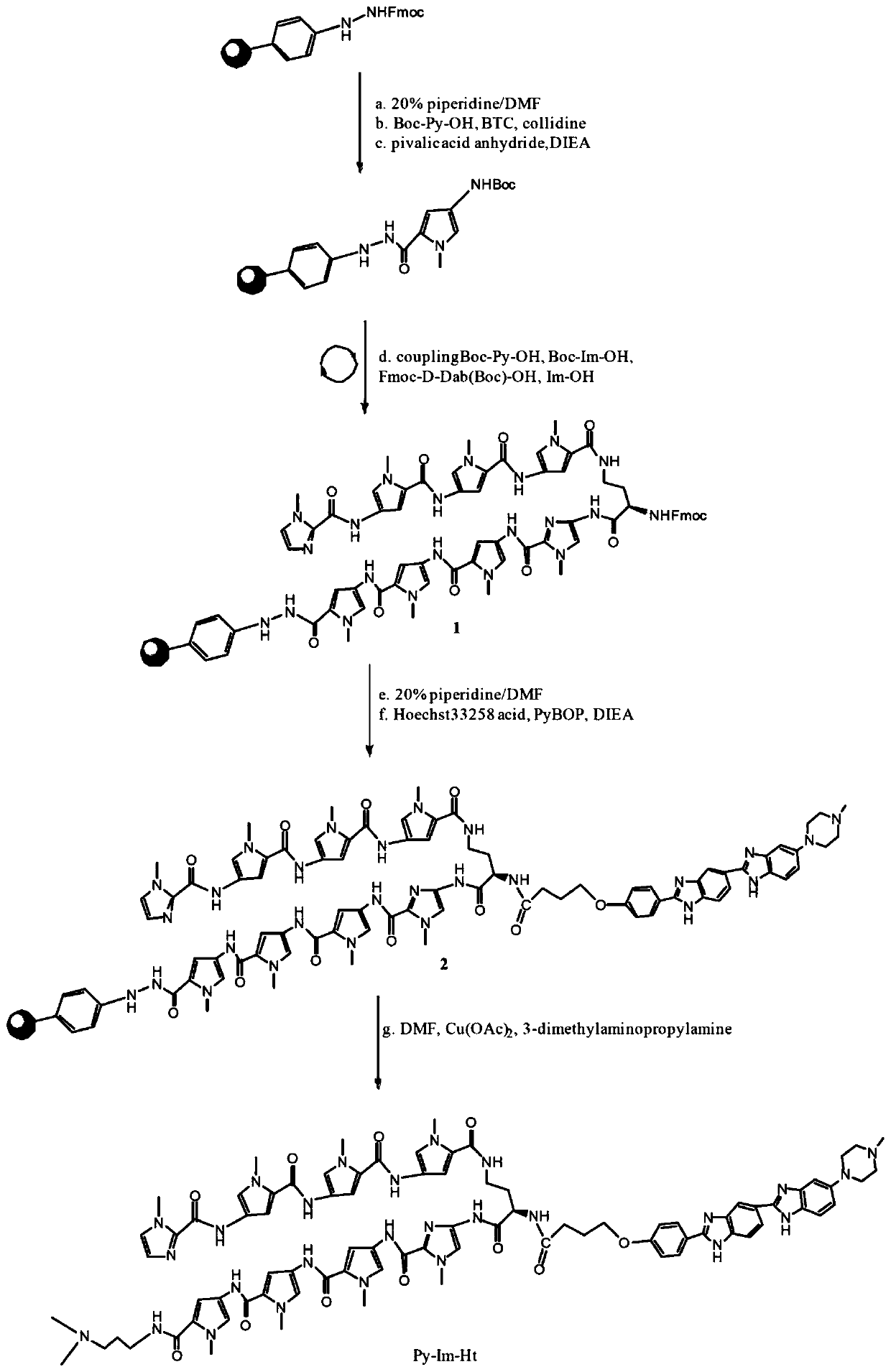
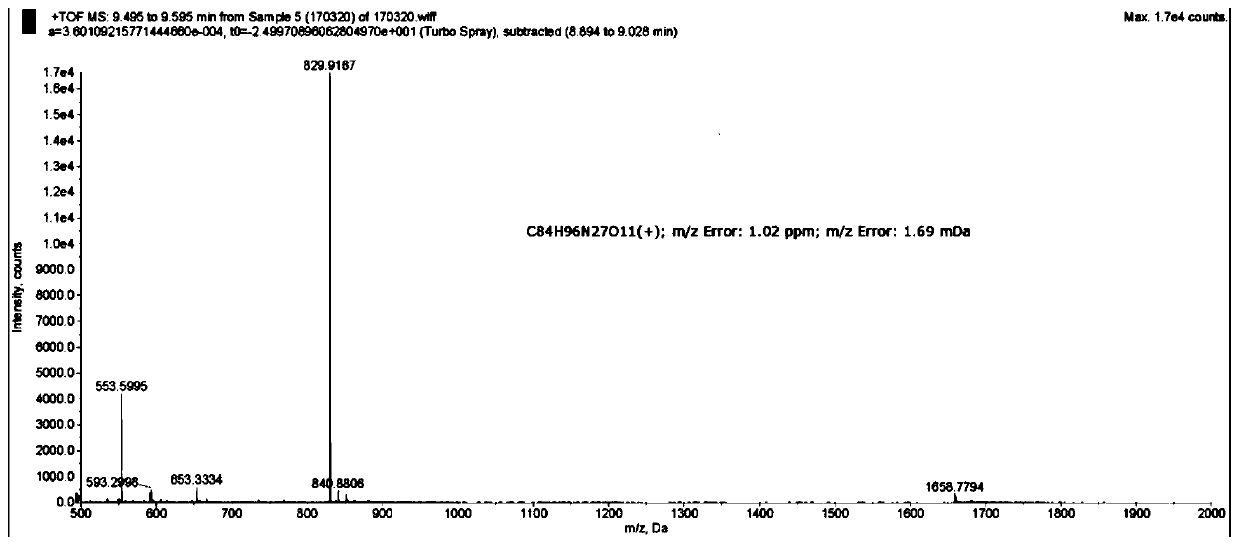
[0051] Embodiment 1: synthetic Py-Im-Ht (synthetic route sees figure 1 )
[0052] 1. Synthesis of Precursor 1
[0053] Add hydrazine resin (0.61mmol / g, 400mg, 0.244mmol) and dichloromethane (3ml) into a 50mL solid phase reactor, and swell the resin for 20min. Extract dichloromethane, add 20% piperidine / DMF solution (3ml) to the resin, blow air for 5min, remove solvent, then add 20% piperidine / DMF solution (3ml), blow air for 5min, remove solvent, The resin was washed with DMF (4x 3 mL). Boc-Py-OH (234mg, 0.976mmol, 4eq.) and triphosgene (BTC, 95mg, 0.322mmol, 0.33eq) were dissolved in 2mL of anhydrous THF, and collidine (collidine, 284μL, 2.928mmol, 12eq.), the reaction immediately produces a large amount of white precipitate, after adding the reaction for 3min, then add 2mL DIEA / DMF solution (5%, v / v), the white precipitate completely disappears, transfer the reaction solution to the In the phenylhydrazine resin of the protective group, N2 was bubbled and mixed, and the ...
Embodiment 2
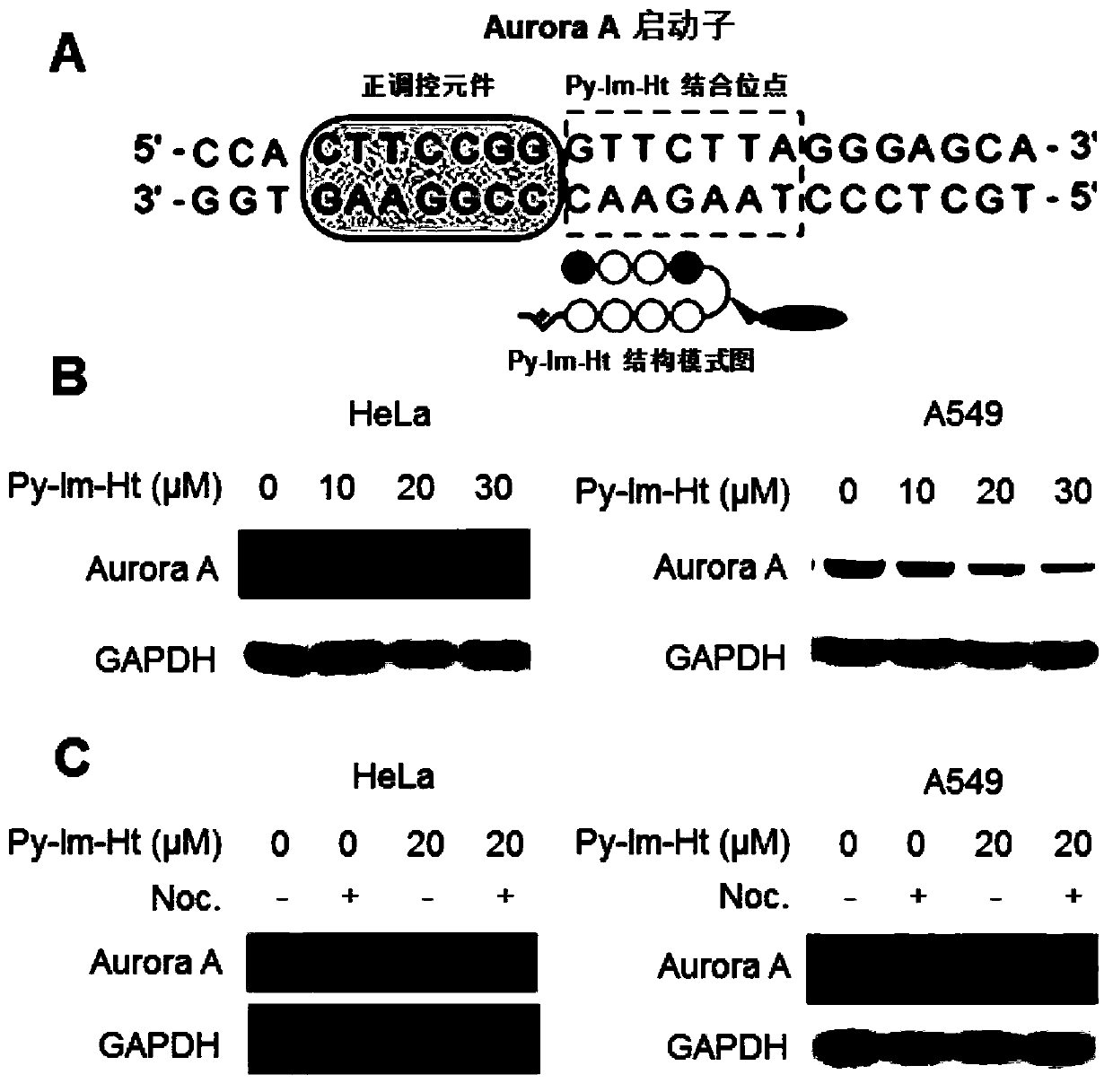
[0069] Embodiment 2: Py-Im-Ht inhibits Aurora A protein expression experiment in tumor cells (HeLa and A549)
[0070]Take Hela cells and A549 cells in the logarithmic growth phase, inoculate 1 mL per well (50,000 cells) in a 12-well plate, and culture them in a 37-degree, 5% carbon dioxide incubator for 24 hours; The original culture medium was added to DMEM high-glucose medium containing different concentrations of Compound 2, so that the drug concentration in each well was 0 μM, 10 μM, 20 μM, and 30 μM; the cells were put back into the incubator and incubated for 72 hours; Digest with 0.25% trypsin at room temperature for 1 min, resuspend the cells in fresh DMEM medium, centrifuge at 3000 rpm for 3 minutes, collect the cells, and wash twice with ice-cold PBS. Remove PBS, centrifuge at 5000rpm for 3 minutes, add RIPA lysate, incubate on ice for 2 hours, centrifuge at 13000rpm at 4 degrees for 15min, collect the supernatant, measure the protein concentration by BCA method, add...
Embodiment 3
[0071] Example 3: Experiment of Py-Im-Ht inhibiting tumor cell proliferation and cell cycle
[0072] Take tumor cells (HeLa, A549 or MDA-MB-231) in the logarithmic growth phase, inoculate 1 mL per well (containing 100,000 cells) in a 6-well plate or 0.1 mL per well (containing 5,000 cells) In a 96-well plate, cultured in a 37°C, 5% carbon dioxide cell incubator for 24 hours; aspirated the original medium, and added it to DMEM high-glucose medium containing different concentrations of Py-Im-Ht; put the cells back into Incubate in an incubator for 48 hours to 72 hours, use a microscope to count the final cell number and the number of cells in each division period or use the CCK-8 kit to measure cell viability, the results are as follows Figure 4 shown. It can be seen that with the prolongation of drug action time, the number of Hela cells and A549 cells decreased significantly ( Figure 4 A&B), indicating that the drug can effectively inhibit the proliferation of tumor cells....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com